Abstract
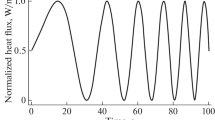
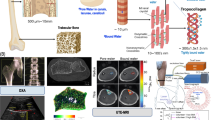
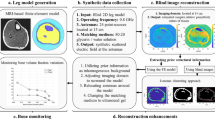
Active infrared thermography (IRT) helps address the constraints of conventional bone density diagnostic tactics, such as various bones requiring different testing methods, unilateral diagnosis, radiation exposure, testing duration, etc. Although active IRT provides promising diagnostic outcomes, it has limited depth penetration into the body, resulting in limited resolution. Despite the fact that numerous post-processing techniques are utilized to boost the intensity penetration of modulated thermal excitation, it is by no means restricted. Consequently, we tend to employ a nanoparticle coating approach in this study. We have coated iron oxide and titanium oxide nanoparticles with two promising excitations, linear frequency modulated (LFM) and Gaussian-weighted linear frequency- modulated (GWLFM). This research aimed to determine the effect of nanoparticle coating in conjunction with GWLFM as it offers increased compression qualities, sensitivity, and resolution. This is accomplished by constructing bone LFM models with varying bone densities and forms. As in the current study, nanoparticles and GWLFM are utilized to attain high depth resolution. Using signal-to-noise ratio, we evaluated diverse coating outcomes with LFM and GWLFM (SNR). In conjunction with GWLFM, titanium- and iron-based nanoparticle coatings are capable of enhancing the depth penetration for bone density measurement, according to our analysis.








Similar content being viewed by others
REFERENCES
Nair, L.S. and Laurencin, C.T., Polymers as biomaterials for tissue engineering and controlled drug delivery, Adv. Biochem. Eng. Biotechnol., 2006, vol. 102, pp. 47–90. https://doi.org/10.1007/b13724
Hench, L.L., Biomaterials: A forecast for the future, Biomaterials, 1998, vol. 19, no. 16, pp. 1419–1423. https://doi.org/10.1016/s0142-9612(98)00133-1
Nassar, E.J., et al., Biomaterials and sol–gel process: A methodology for the preparation of functional materials, in: Biomaterials Science and Engineering, Pignatello, R., Ed., London: IntechOpen, 2011.
Agrawal, C. M., Reconstructing the human body using biomaterials, J. Med., 1998, vol. 50, no. 1, pp. 31–35. https://doi.org/10.1007/s11837-998-0064-5
Bronzino, J.D., The Biomedical Engineering Handbook, Boca Raton: CRC Press, 2000, 2nd Ed.
Yoruc, A.B.H. and Sener, B.C., Biomaterials, in: A Roadmap of Biomedical Engineers and Milestones, Kara, S., Ed., 2012.
Campoccia, D., Montanaro, L., and Arciola, C.R., A review of the biomaterials technologies for infection-resistant surfaces, Biomaterials, 2013, vol. 34 (34), pp. 8533–8554. https://doi.org/10.1016/j.biomaterials.2013.07.089
Chaloupka, K., Malam, Y., and Seifalian, A.M., Nanosilver as a new generation of nanoproduct in biomedical applications, Trends Biotechnol., 2010, vol. 28, no. 11, pp. 580–588. https://doi.org/10.1016/j.tibtech.2010.07.006
Adedoyin, A.A. and Ekenseair, A.K., Biomedical applications of magnetoresponsive scaffolds, Nano Res., 2018, vol. 11, no. 10, pp. 5049–5064. https://doi.org/10.1007/s12274-018-2198-2
Maldague, X.P.V., Theory and Practice of Infrared Technology for Nondestructive Testing, Hoboken: Wiley, 2001.
Ringermacher, H.I., et al., Flash quenching for high resolution thermal depth imaging, Proc. AIP, 2004, vol. 700, pp. 477–481.
Djupkep, F.B.D., Maldague, X., Bendada, A., and Bison, P., Analysis of a new method of measurement and visualization of indoor conditions by infrared thermography, Rev. Sci. Instrum., 2013, vol. 84, no. 8, p. 084906. https://doi.org/10.1063/1.4818919
Nayak, S., Edwards, D.L., Saleh, A.A., and Greenspan, S.L., Systematic review and meta-analysis of the performance of clinical risk assessment instruments for screening for osteoporosis or low bone density, Osteoporosis Int., 2015, vol. 26, no. 5, pp. 1543–1554. https://doi.org/10.1007/s00198-015-3025-1
Dua, G. and Mulaveesala, R., Infrared thermography for detection and evaluation of bone density variations by non-stationary thermal wave imaging, Biomed. Phys. Eng. Express, 2017, vol. 3, no. 1, p. 017006. https://doi.org/10.1088/2057-1976/aa5b4d
Sharma, A., Mulaveesala, R., Dua, G., and Kumar, N., Linear frequency modulated thermal wave imaging for estimation of osteoporosis: An analytical approach, Electron. Lett., 2020, vol. 56, no. 19, pp. 1007–1010. https://doi.org/10.1049/el.2020.0671
Feng, L., et al., Lock-in thermography and its application in nondestructive evaluation, Infrared Laser Eng., 2010, vol. 39, pp. 1121–1123.
Almond, D.P. and Pickering, S.G., An analytical study of the pulsed thermography defect detection limit, J. Appl. Phys., 2012, vol. 1, p. 093510.
Levy, A., Dayan, A., Ben-David, M., and Gannot, I., A new thermography-based approach to early detection of cancer utilizing magnetic nanoparticles theory simulation and in vitro validation, Nanomed. Nanotechnol. Biol. Med., 2010, vol. 6, no. 6, pp. 786–796. https://doi.org/10.1016/j.nano.2010.06.007
Ohashi, Y. and Uchida, I., Applying dynamic thermography in the diagnosis of breast cancer, IEEE Eng. Med. Biol. Mag. Quarterly Mag. Eng. Med. Biol. Soc., 2000, vol. 19, no. 3, pp. 42–51. https://doi.org/10.1109/51.844379
Raverkar, P., Siddiqui, J. A., Kulkarni, A., and Mulaveesala, R., Corrosion detection in mild steel using effective post-processing technique for modulated thermal imaging: A numerical study, IEEE Int. Conf. Technol. Res. Innovation Betterment Soc. (TRIBES), Raipur, 2021, pp. 1–4.
Mulaveesala, R. and Ghali, V.S., Cross-correlation based approach for thermal nondestructive characterization of carbon fiber reinforced plastics, Insight Nondestr. Test. Cond. Monit., 2011, vol. 53, no. 1, pp. 34–36. https://doi.org/10.1784/insi.2011.53.1.34
Ghali, V.S., Jonnalagadda, N., and Mulaveesala, R., Three dimensional pulse compression for infrared nondestructive testing, IEEE Sens. J., 2009, vol. 9, no. 7, pp. 832–833. https://doi.org/10.1109/JSEN.2009.2024042
Siddiqui, J.A., et al., Modelling of the frequency modulated thermal wave imaging process through the finite element method for nondestructive testing of a mild steel sample, Insight Nondestr. Test. Cond. Monit., 2015, vol. 57, pp. 266–268.
Mulaveesala, R., Vaddi, J.S., and Singh, P., Pulse compression approach to infrared nondestructive characterization, Rev. Sci. Instrum., 2008, vol. 79, no. 9, p. 094901. https://doi.org/10.1063/1.2976673
Dua, G., Mulaveesala, R., and Siddique, J.A., Effect of spectral shaping on defect detection in frequency modulated thermal wave imaging, J. Opt., 2015, vol. 17, no. 2, pp. 1–5. https://doi.org/10.1088/2040-8978/17/2/025604
Sharma, A., Mulaveesala, R., and Arora, V., Novel analytical approach for estimation of thermal diffusivity and effusivity for detection of osteoporosis, IEEE Sens. J., 2020, vol. 20, no. 11, pp. 6046–6054. https://doi.org/10.1109/JSEN.2020.2973233
Davidson, S.R.H., Heat transfer in bone during drilling, Science Thesis, University of Toronto, 1999.
Zhang, L., Yu, W., Zhu, D., Xie, H., and Huang, G., Enhanced thermal conductivity for nanofluids containing silver nanowires with different shapes, J. Nanomater., 2017, pp. 1–6. https://doi.org/10.1155/2017/5802016
Mulaveesala, R. and Tuli, S., Theory of frequency modulated thermal wave imaging for nondestructive subsurface defect detection, Appl. Phys. Lett., 2006, vol. 89, no. 19, p. 191913. https://doi.org/10.1063/1.2382738
Arora, V. and Mulaveesala, R., Pulse compression with Gaussian weighted chirp modulated excitation for infrared thermal wave imaging, Prog. Electromagn. Res. Lett., 2014, vol. 44, pp. 133–137. https://doi.org/10.2528/PIERL13111301
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dass, S., Siddiqui, J.A. & Mulaveesala, R. Applicability of Nanoparticle Coating in Bone Density Evaluation Using Gaussian-Weighted Linear Frequency-Modulated Thermal Wave Imaging. Russ J Nondestruct Test 59, 228–239 (2023). https://doi.org/10.1134/S106183092260109X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106183092260109X