Abstract
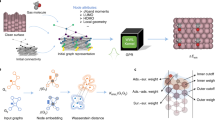
This manuscript presents a study of the applicability of machine learning methods for the problem of binding energy approximation of carbon monoxide adsorbates on the surface of a palladium nanoparticle. Machine learning algorithms were trained using a set of structures that represent models of CO interaction with different parts of the Pd55 nanocluster with a variable distance from the molecule to the surface, for which the energy was calculated using the density functional theory methods. For structures that make up a training set, the radial distribution functions were calculated. Using these functions and their segments as descriptors, the effectiveness of various machine learning algorithms, such as “gradient boosting,” “ridge regression,” “extra trees,” and “support vector machines” for calculating the binding energy, was tested. Based on three different metrics, it was found that the error in determining the binding energy was the smallest when using the “support vector machines”: the mean absolute error was 0.093 eV. The efficiency of various individual sections of the distribution function used as descriptors was compared. It was found that it is crucial to take into account the part of the radial distribution function from 1.5 to 2.5 Å for a correct approximation of the energy.





Similar content being viewed by others
REFERENCES
D. Pakhare and J. Spivey, Chem. Soc. Rev. 43, 7813 (2014). https://doi.org/10.1039/C3CS60395D
V. Pareek, A. Bhargava, R. Gupta, N. Jain, and J. Panwar, Adv. Sci. Eng. Med. 9, 527 (2017). https://doi.org/10.1166/asem.2017.2027
K. Kinoshita, J. Electrochem. Soc. 137, 845 (2017). https://doi.org/10.1149/1.2086566
S. Rojluechai, S. Chavadej, J. W. Schwank, and V. Meeyoo, Catal. Commun. 8, 57 (2007). https://doi.org/10.1016/j.catcom.2006.05.029
C. J. DeSantis, A. A. Peverly, D. G. Peters, and S. E. Skrabalak, Nano Lett. 11, 2164 (2011). https://doi.org/10.1021/nl200824p
C. Sun, Z. Cao, J. Wang, L. Lin, and X. Xie, New J. Chem. 43, 2567 (2019). https://doi.org/10.1039/C8NJ05152F
S. K. Vatti, K. K. Ramaswamy, and V. Balasubramanaian, J. Adv. Nanomat. 2, 127 (2017). https://doi.org/10.22606/jan.2017.22006
B. R. Cuenya, Thin Solid Films 518, 3127 (2010). https://doi.org/10.1016/j.tsf.2010.01.018
A. A. Tereshchenko, V. A. Polyakov, A. A. Guda, A. N. Bulgakov, A. L. Tarasov, L. M. Kustov, V. V. Butova, A. L. Trigub, and A. V. Soldatov, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 14, 447 (2020). https://doi.org/10.1134/S1027451020030180
I. V. Yudanov, R. Sahnoun, K. M. Neyman, N. Rösch, J. Hoffmann, S. Schauermann, V. Johanek, H. Unterhalt, G. Rupprechter, and J. Libuda, J. Chem. Phys. B 107, 255 (2003). https://doi.org/10.1021/jp022052b
X. Wang, G. Wu, N. Guan, and L. Li, Appl. Catal., B 115, 7 (2012). https://doi.org/10.1016/j.apcatb.2011.12.011
Q. Fan, S. He, L. Hao, X. Liu, Y. Zhu, S. Xu, and F. Zhang, Sci. Rep. 7, 1 (2017). https://doi.org/10.1038/srep42172
L. L. Sheu, Z. Karpinski, and W. M. Sachtler, J. Chem. Phys. 93, 4890 (1989). https://doi.org/10.1021/j100349a042
A. Tereshchenko, A. Guda, V. Polyakov, Y. Rusalev, V. Butova, and A. Soldatov, Analyst 145, 7534 (2020). https://doi.org/10.1039/D0AN01303J
B. R. Cuenya and F. Behafarid, Surf. Sci. Rep. 70, 135 (2015). https://doi.org/10.1016/j.surfrep.2015.01.001
C. D. Rankine, M. M. Madkhali, and T. J. Penfold, J. Chem. Phys. A 124, 4263 (2020). https://doi.org/10.1021/acs.jpca.0c03723
J. Timoshenko, D. Lu, Y. Lin, and A. I. Frenkel, J. Phys. Chem. Lett. 8, 5091 (2017). https://doi.org/10.1021/acs.jpclett.7b02364
J. Timoshenko, A. Anspoks, A. Cintins, A. Kuzmin, J. Purans, and A. I. Frenkel, Thin Solid Films 120, 225502 (2018). https://doi.org/10.1103/PhysRevLett.120.225502
S. A. Tupy, A. M. Karim, C. Bagia, W. Deng, Y. Huang, D. G. Vlachos, and J. G. Chen, ACS Catal. 2, 2290 (2012). https://doi.org/10.1021/cs3004227
J. L. Lansford and D. G. Vlachos, Nat. Commun. 11, 1513 (2020). https://doi.org/10.1038/s41467-020-15340-7
F. Calle-Vallejo, J. I. Martínez, J. M. García-Lastra, P. Sautet, and D. Loffreda, Angew. Chem., Int. Ed. 53, 8316 (2014). https://doi.org/10.1002/anie.201402958
I. Takigawa and K. Shimizu, RSC Adv. 6, 52587 (2016). https://doi.org/10.1039/C6RA04345C
R. Gasper, H. Shi, and A. Ramasubramaniam, J. Chem. Phys. C 121, 5612 (2017). https://doi.org/10.1021/acs.jpcc.6b12800
G. Kresse and J. Furthmüller, Phys. Rev. B 54, 11169 (1996).
G. Kresse and J. Furthmüller, Comp. Mater. Sci. 6, 15 (1996).
S. Grimme, J. Comput. Chem. 27, 1787 (2006).
J. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 80, 891 (1998).
C. Lamberti, A. Zecchina, E. Groppo, and S. Bordiga, Chem. Soc. Rev. 39, 4951 (2010). https://doi.org/10.1039/C0CS00117A
Funding
The study was carried out with the financial support of the Ministry of Science and Higher Education (agreement no. 075-15-2021-1363). Andrei Tereshchenko thanks the Russian Foundation for Basic Research (research project no. 20-32-90 048) for financial support of experimental studies to verify the results of theoretical modeling by FTIR spectroscopy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tereshchenko, A.A., Pashkov, D.M., Guda, A.A. et al. Application of Machine Learning Methods to Approximate the Binding Energy of CO Molecules on the Surface of Pd Nanoparticles. J. Surf. Investig. 16, 901–908 (2022). https://doi.org/10.1134/S1027451022050366
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451022050366