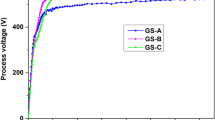
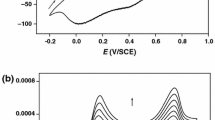
Abstract
Corrosion resistant black anodic passive film, composed of crystalline ZnO structures, was successfully fabricated on a galvanized surface by room-temperature and low-voltage anodization route using an eco-friendly and cost-effective mixed electrolyte without any surface treatments. In addition, the properties and corrosion resistance of ZnO passive films fabricated by other methods were compared with those of anodic ZnO films. It was found that the anti-corrosive properties of ZnO films were significantly influenced by the chemical composition of the surface, surface roughness and wetting behaviour. Experimental results showed that hydrophilic anodic films (surface energy γ = 17.3 mN/m and adhesion Wst = 70.6 mN/m) could effectively enhance corrosion resistance of galvanized steel. In addition, it was found that film porosity and thickness also played a critical role in the corrosion resistance of anodic ZnO films. The porosity and thickness of the anodic films increased with anodizing time, and a dense anodic film with a thickness of 668 nm was fabricated at 10 V for 30 min in a mixed electrolyte, and this one showed the best corrosion resistance.



Similar content being viewed by others
REFERENCES
Gh. B. Darband, A. Afshar, and A. Aliabadi, Surf. Coat. Technol. 306, 497 (2016). https://doi.org/10.1016/j.surfcoat.2015.12.089
T. Naing, S. Janudom, V. Rachpech, N. Mahathaninwonga, and S. Thiwong, Key Eng. Mater. 803, 45 (2019). doi 10.4028/www.scientific.net/KEM.803.45
L. Hernández and S. L. Rodriguez Reyna, Int. J. Corros. 2012, 368130 (2012). https://doi.org/10.1155/2012/368130
M. Sánchez, M. C. Alonso, P. Cecílio, M. F. Montemor, and C. Andrade, Cem. Concr. Compos. 28, 256 (2006). https://doi.org/10.1016/j.cemconcomp.2006.01.004
S. Jegannathan, T. S. N. Sankara Narayanan, K. Ravichandran, and S. Rajeswari, Electrochim. Acta 51, 247 (2005). https://doi.org/10.1016/j.electacta.2005.04.020
D. Wang and G. P. Bierwagen, Prog. Org. Coat. 64, 327 (2009). https://doi.org/10.1016/j.porgcoat.2008.08.010
K. S. Aneja, S. Bohm, A. S. Khanna, and H. L. M. Bohm, Nanoscale 7, 17879 (2015). https://doi.org/10.1039/C5NR04702A
S. J. Kim, J. Lee, and J. Choi, Electrochim. Acta 53, 7941 (2008). https://doi.org/10.1016/j.electacta.2008.06.006
D. Landolt, Corrosion and Surface Chemistry of Metals (EPFL, New York, 2007). https://doi.org/10.1201/9781439807880
G. S. Huang, X. L. Wu, Y. C. Cheng, J. C. Shen, A. P. Huang, and P. K. Chu, Appl. Phys. A 86, 463 (2007). https://doi.org/10.1007/s00339-006-3778-7
M.-C. Huang, T. Wang, B.-J. Wu, J.-C. Lin, and C.-C. Wu, Appl. Surf. Sci. 360, 442 (2016). https://doi.org/10.1016/j.apsusc.2015.09.174
S. He, M. Zheng, L. Yao, X. Yuan, M. Li, L. Ma, and W. Shen, Appl. Surf. Sci. 256, 2557 (2010). https://doi.org/10.1016/j.apsusc.2009.10.104
A. Ramirez-Canon, D. O. Miles, P. J. Cameron, and D. Mattia, RSC Adv. 3, 25323 (2013). https://doi.org/10.1039/C3RA43886D
P. Gao and Z. L. Wang, J. Phys. Chem. B 106, 12653 (2002). https://doi.org/10.1021/jp0265485
Z. W. Pan, Z. R. Dai, and Z. L. Wang, Science, 291, 1947 (2001). https://doi.org/10.1126/science.1058120
H.-S. Goh, R. Adnan, and M. A. Farrukh, Turk. J. Chem. 35, 375 (2011). https://doi.org/10.3906/kim-1010-742
S. Sreekantan, L. R. Gee, and Z. Lockman, J. Alloys Compd. 476, 513 (2009). https://doi.org/10.1016/j.jallcom.2008.09.044
S. A. Ajeel and B. Ahmed, Eng. Technol. J. A 35, 961 (2017).
T. H. Naing, S. Janudom, V. Rachpech, N. Mahathaninwong, and S. Thiwong, Mater. Res. Express 6, 116415 (2019). https://doi.org/10.1088/2053-1591/ab45b2
T. H. Naing, V. Rachpech, S. Janudom, and N. Mahathaninwong, J. Coat. Technol. Res. 17, 1537 (2020). https://doi.org/10.1007/s11998-020-00372-x
L. Zaraska, K. Mika, K. E. Hnida, M. Gajewska, T. Łojewski, M. Jaskuła, and G. D.Sulka, Mater. Sci. Eng. B 226, 94 (2017). https://doi.org/10.1016/j.mseb.2017.09.003
S. Mridha and D. Basak, Mater. Res. Bull. 42, 875 (2007). https://doi.org/10.1016/j.materresbull.2006.08.019
E. Rocca, D. Veys-Renaux, and K. Guessoum, J. Electroanal. Chem. 754, 125 (2015). .https://doi.org/10.1016/j.jelechem.2015.06.021
M.-C. Huang, T. Wang, B.-J. Wu, J.-C. Lin, and C.-C. Wu, Appl. Surf. Sci. 360, 442 (2016). https://doi.org/10.1016/j.apsusc.2015.09.174
A. B. Radwan, A. M. Abdullah, and N. A. Alnuaimi, Corros. Rev. 36, 127 (2018). https://doi.org/10.1515/corrrev-2017-0012
R. N. Wenzel, Ind. Eng. Chem. 28, 988 (1936). https://doi.org/10.1021/ie50320a024
M. D. L. Balela, R. A. Acedera, C. L. I. Flores, and C. M. O. Pelicano, Surf. Coat. Technol. 340, 199 (2018). https://doi.org/10.1016/j.surfcoat.2018.02.055
W. Wu, M. Chen, S. Liang, X. Wang, J. Chen, and F. Zhou, J. Colloid Interface Sci. 326, 478 (2008). https://doi.org/10.1016/j.jcis.2008.06.041
A. B. D. Cassie and S. Baxter, Trans. Faraday Soc. 40, 546 (1944). https://doi.org/10.1039/TF9444000546
L. Wu, J. Liu, M. Yu, S. Li, H. Liang, and M. Zhu, Int. J. Electrochem. Sci. 9, 5012 (2014).
R. P. S. Chakradhar and V. Dinesh Kumar, Spectrochim. Acta, Part A 94, 352 (2012). https://doi.org/10.1016/j.saa.2012.03.079
A. B. Gurav, S. S. Latthe, R. S. Vhatkar, J.-G. Lee, D.-Y. Kim, J.-J. Park, and S. S. Yoon, Ceram. Int. 40, 7151 (2014). https://doi.org/10.1016/j.ceramint.2013.12.052
J. Dong, Z. Liu, J. Dong, D. Ariyanti, Z. Niu, S. Huang, W. Zhang, and W. Gao, RSC Adv. 6, 72968 (2016). https://doi.org/10.1039/C6RA16995C
A. Achour, M. A. Soussou, K. A. Aissa, M. Islam, N. Barreau, E. Faulques, L. L. Brizoual, M. A. Djouadi, and M. Boujtita, Thin Solid Films 571, 168 (2014). https://doi.org/10.1016/j.tsf.2014.10.061
S. Xu and Z. L. Wang, Nano Res. 4 (11), 1013 (2011). https://doi.org/10.1007/s12274-011-0160-7
S. E. Pust, J.-P. Becker, J. Worbs, S. O. Klemm, K. J. J. Mayrhofer, and J. Hüpkes, J. Electrochem. Soc. 158, D413 (2011). https://doi.org/10.1149/1.3583636
J. Hüpkes, J. I. Owen, S. E. Pust, and E. Bunte, ChemPhysChem 13, 66 (2012). https://doi.org/10.1002/cphc.201100738
J.-P. Becker, S. E. Pust, and J. Hüpkes, Electrochim. Acta 112, 976 (2013). https://doi.org/10.1016/j.electacta.2013.04.167
E. Vazirinasab, R. Jafari, and G. Momen, Surf. Coat. Technol. 341, 40 (2018). https://doi.org/10.1016/j.surfcoat.2017.11.053
J. Ou, M. Liu, W. Li, F. Wang, M. Xue, and C. Li, Appl. Surf. Sci. 258, 4724 (2012). https://doi.org/10.1016/j.apsusc.2012.01.066
T. Liu, S. Chen, S. Cheng, J. Tian, X. Chang, and Y. Yin, Electrochim. Acta 52 (28), 8003 (2007). https://doi.org/10.1016/j.electacta.2007.06.072
W. Xu, X. Shi, and S. Lu, Mater. Chem. Phys. 129, 1042 (2011). https://doi.org/10.1016/j.matchemphys.2011.05.053
L. Chai, X. Yu, Z. Yang, Y. Wang, and M. Okido, Corros. Sci. 50, 3274 (2008). https://doi.org/10.1016/j.corsci.2008.08.038
G.-L. Song and Z. Shi, Corros. Sci. 85, 126 (2014). https://doi.org/10.1016/j.corsci.2014.04.008
A. Maciej, A. Wadas, M. Sowa, R. Socha, G. Dercz, M. Rabe, and W. Simka, Corros. Sci. 158, 108107 (2019). https://doi.org/10.1016/j.corsci.2019.108107
Funding
The authors acknowledge Center of Excellence in Mining and Materials Engineering (CEMME), Faculty of Engineering, Prince of Songkla University (PSU). Also sincere thanks go to the Prince of Songkla University (Contract nos. ENG610407S and ENG6201029S) for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Naing, T.H., Janudom, S., Rachpech, V. et al. Black Anodic ZnO Film on Galvanized Steel Using Mixed Electrolyte of Ca(OH)2–KOH–NaOH. J. Surf. Investig. 15 (Suppl 1), S104–S111 (2021). https://doi.org/10.1134/S1027451022020161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451022020161