Abstract
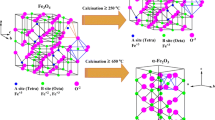
In this paper, we decomposed the FeSO4 at the presence of benzoic acid with weight ratio 1 : 1 at two different temperatures 500 and 600°C. The as-synthesized products were characterized by Fourier Transform Infrared (FT-IR) spectroscopy, powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). FT-IR and XRD results confirmed that the as-prepared product at 500°C is a mixture of intermediate compounds, while the as-prepared product at 600°C is obviously pure with high degree of crystallinity of hematite (α-Fe2O3) nanoparticles. TEM images show that shape, size and morphology of the as-prepared products depend on the calcination temperature.



Similar content being viewed by others
REFERENCES
H. J. Zhang, F. N. Meng, L. Z. Liu, and Y. J. Chen, J. Alloys Compd. 774, 1181 (2019).
M. Qiu, R. Wang, and X. Qi, J. Taiwan Inst. Chem. Eng. 102, 394 (2019).
A. Kusior, K. Michalec, P. Jelen, and M. Radecka, Appl. Surf. Sci. 476, 342 (2019).
X. Wang, J. Zhang, Z. Wang, Y. Wang, M. Vujanovic, P. Li, and H. Tan, J. Enviroment. Manage. 236, 420 (2019).
S. Hu, L. Jiang, B. Wang, and Y. Ma, Int. J. Hydrogen Energy 44, 13214 (2019).
Z. Li, Y. Mao, Q. Tian, W. Zhang, and L. Yang, J. Alloys Compd. 784, 125 (2019).
S. Yao, Z. Shi, and X. Zhang, J. Alloys Compd. 794, 333 (2019).
M. Wang, T. Hou, Z. Shen, X. Zhao, and H. Ji, Sens. Actuators, B 292, 171 (2019).
Z. Huang, L. Qin, Z. Xu, W. Chen, F. Xing, and J. Han, J. Energy Inst. 92, 835 (2019).
J. Liu, H. Yang, and X. Xue, CrystEngComm 21, 1097 (2019).
D. Li, R. Xu, Y. Jia, P. Ning, and K. Li, Chem. Phys. Lett. 731, 1336623 (2019).
J. R. Jesus, R. J. S. Lima, K. O. Moura, J. G. S. Duque, and C. T. Meneses, Ceram. Int. 44, 3585 (2018).
G. Huang, E. He, Z. Wang, H. Fan, J. Shangguan, E. Croiset, and Z. Chen, Ind. Eng. Chem. Res. 54, 8469 (2015).
C. Hao, F. Feng, X. Wang, M. Zhou, Y. Zhao, C. Ge, and K. Wang, RSC Adv. 5, 21161 (2015).
D. M. S. N. Dissanayake, M. M. M. G. P. G. Mantilaka, T. C. Palohawadana, G. T. D. Chandrakumara, R. T. De Silva, H. M. G. A. Pitawala, K. M. Nalin de Silva, and G. A. J. Amaratunga, RSC Adv. 9, 21249 (2019).
H. Cui, Y. Liu, and W. Ren, Adv. Powder Technol. 24, 93 (2013).
X. Zhang, Y. Niu, X. Meng, Y. Li, and J. Zhao, CrystEngComm 15, 8166 (2013).
V. Petricek, M. Dusek, and L. Palatinus, Z. Kristallogr. 229, 345 (2014).
R. W. Cheary and A. A. Coelho, J. Appl. Crystallogr. 31, 862 (1998).
ACKNOWLEDGMENTS
We are grateful to the Golestan University for financial support of this work. XRD and TEM analysis were supported by the project 18-10504S of the Czech Science Foundation using instruments of the ASTRA lab established within the Operation program Prague Competitiveness e project CZ.2.16/3.1.00/2451.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliakbar Dehno Khalaji, Mousavi, S.M., Jarosova, M. et al. The Effect of Calcination Temperature on the Morphology, Size and the Crystalline Phase of the As-Synthesized Products by Direct Calcination of FeSO4 at the Presence of Benzoic Acid. J. Surf. Investig. 14, 1191–1194 (2020). https://doi.org/10.1134/S1027451020060051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451020060051