Abstract
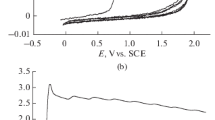
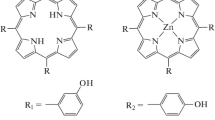
Electrochemical polymerization of 3,4-ethylenedioxythiophene (EDOT) in the presence of water-soluble sodium salt of zinc octa(3′,5′-dicarboxyphenoxy)phthalocyaninate containing 16 ionogenic carboxylate groups is studied. By using electrochemical and spectral methods of monitoring the course of the electrosynthesis the EDOT polymerization in the presence of phthalocyaninate is shown to proceed at higher rate compared to that in the presence of low-molecular electrolyte (sodium acetate). The electropolymerization acceleration is discussed in terms of templating effect of locally-ordered carboxylate groups of phthalocyaninate in analogy with the EDOT template electropolymerization in the presence of polyelectrolytes. Electronic and chemical structures, morphology, spectroelectrochemical and sensor properties (with respect to ammonia) of the PEDOT composite films obtained in the presence of water-soluble phthalocyaninate are studied for the first time.











Similar content being viewed by others
REFERENCES
John, R. Reynolds, Barry, C. and Thompson, T.A.S., Handbook of Conducting Polymers, Fourth Edition—2 Volume Set; Reynolds, J.R., Thompson, B.C., and Skotheim, T.A., Eds.; CRC Press, 2019; ISBN 9781351660235
Lange, U., Roznyatovskaya, N.V., and Mirsky, V.M., Conducting polymers in chemical sensors and arrays, Anal. Chim. Acta, 2008, vol. 614, p. 1.https://doi.org/10.1016/j.aca.2008.02.068
Borgohain, R., Kumar Boruah, P., and Baruah, S., Heavy-metal ion sensor using chitosan capped ZnS quantum dots. Sensors Actuators, B Chem., 2016, vol. 226, p. 534.https://doi.org/10.1016/j.snb.2015.11.118
Sizun, T., Patois, T., Bouvet, M., and Lakard, B., Microstructured electrodeposited polypyrrole-phthalocyanine hybrid material, from morphology to ammonia sensing, J. Mater. Chem., 2012, vol. 22, p. 25246.https://doi.org/10.1039/c2jm35356c
Patois, T., Sanchez, J.B., Berger, F., Fievet, P., Segut, O., Moutarlier, V., Bouvet, M., and Lakard, B., Elaboration of ammonia gas sensors based on electrodeposited polypyrrole—Cobalt phthalocyanine hybrid films, Talanta, 2013, vol. 117, p. 45.https://doi.org/10.1016/j.talanta.2013.08.047
Guo, Y., Hao, X., Tao, Y., Zhang, C., and Cheng, H., Preparation, characterizations and electrochromic properties of copolymers containing 5, 10, 15, 20-tetra(thienyl) porphyrin and thiophene derivatives, Synth. Met., 2019, vol. 258, p. 116202.https://doi.org/10.1016/j.synthmet.2019.116202
Solis, C., Baigorria, E., Milanesio, M.E., Morales, G., Durantini, E.N., Otero, L., and Gervaldo, M., Electrochemical polymerization of EDOT modified Phthalocyanines and their applications as electrochromic materials with green coloration, and strong absorption in the Near-IR, Electrochim. Acta, 2016, vol. 213, p. 594.https://doi.org/10.1016/j.electacta.2016.07.086
Pari, M. and Reddy, K.R.V., A Facile Cobalt(II) Tetra Amino Phthalocyanine Ingrained Poloy Aniline (PANI) Nano-fiber Film Layer Based Electrode Material for Amperometric Determination of Thiocyanate, J. Inorg. Organomet. Polym. Mater., 2020, vol. 30, p. 3511.https://doi.org/10.1007/s10904-020-01515-8
Milczarek, G., Self-doped polyaniline films prepared by electropolymerization in the presence of sulfonated nickel phthalocyanine, Thin Solid Films, 2009, vol. 517, p. 6100.https://doi.org/10.1016/j.tsf.2009.04.055
Vijayanathan, V., Venkatachalam, S., and Krishnamurthy, V.N., Oligomeric metallo phthalocyanine-incorporated conducting polymers, Synth. Met., 2000, vol. 114, p. 273.https://doi.org/10.1016/s0379-6779(00)00247-2
Vijayanathan, V., Venkatachalam, S., and Krishnamurthy, V.N., Electroactive polymeric thin film based on polypyrrole incorporating metallophthalocyanine polymer, Polymer (Guildf), 1993, vol. 34, p. 1095.https://doi.org/10.1016/0032-3861(93)90235-3
Muthuraman, G., Shim, Y.B., Yoon, J.H., and Won, M.S., Simultaneous immobilization of cobalt tetrasulfonated phthalocyanine during electropolymerization of pyrrole in presence of surfactants: A study of film morphology and its conductivity, Synth. Met., 2005, vol. 150, p. 165.https://doi.org/10.1016/j.synthmet.2005.02.002
Gribkova, O., Kabanova, V., Tverskoy, V., and Nekrasov, A., Comparison of Optical Ammonia-Sensing Properties of Conducting Polymer Complexes with Polysulfonic Acids, Chemosensors, 2021, vol. 9, p. 206.https://doi.org/10.3390/chemosensors9080206
Ismail, A.H., Mohd Yahya, N.A., Yaacob, M.H., Mahdi, M.A., and Sulaiman, Y., Optical ammonia gas sensor of poly(3,4-polyethylenedioxythiophene), polyaniline and polypyrrole: A comparative study, Synth. Met., 2020, vol. 260, p. 116294.https://doi.org/10.1016/j.synthmet.2020.116294
Lakard, B., Carquigny, S., Segut, O., Patois, T., and Lakard, S., Gas Sensors Based on Electrodeposited Polymers, Metals (Basel), 2015, vol. 5, p. 1371.https://doi.org/10.3390/met5031371
Kabanova, V., Gribkova, O., and Nekrasov, A., Poly(3,4-ethylenedioxythiophene) electrosynthesis in the presence of mixtures of flexible-chain and rigid-chain polyelectrolytes, Polymers (Basel), 2021, vol. 13, p. 3866.https://doi.org/10.3390/polym13223866
Liu, W., Jensen, T.J., Fronczek, F.R., Hammer, R.P., Smith, K.M., and Vicente, M.G.H., Synthesis and cellular studies of nonaggregated water-soluble phthalocyanines, J. Med. Chem., 2005, vol. 48, p. 1033.https://doi.org/10.1021/jm049375b
MacHacek, M., Kollár, J., Miletin, M., Kučera, R., Kubát, P., Simunek, T., Novakova, V., and Zimcik, P., Anionic hexadeca-carboxylate tetrapyrazinoporphyrazine: Synthesis and in vitro photodynamic studies of a water-soluble, non-aggregating photosensitizer, RSC Adv., 2016, vol. 6, p. 10064.https://doi.org/10.1039/c5ra25881b
Pigani, L., Heras, A., Colina, Á., Seeber, R., and López-Palacios, J., Electropolymerisation of 3,4-ethylenedioxythiophene in aqueous solutions, Electrochem. commun., 2004, vol. 6, p. 1192.https://doi.org/10.1016/j.elecom.2004.09.021
Gribkova, O.L., Iakobson, O.D., Nekrasov, A.A., Cabanova, V.A., Tverskoy, V.A., and Vannikov, A.V., The influence of polyacid nature on poly(3,4-ethylenedioxythiophene) electrosynthesis and its spectroelectrochemical properties, J. Solid State Electrochem., 2016, vol. 20, p. 2991.https://doi.org/10.1007/s10008-016-3252-1
Zozoulenko, I., Singh, A., Singh, S.K., Gueskine, V., Crispin, X., and Berggren, M., Polarons, Bipolarons, and Absorption Spectroscopy of PEDOT, ACS Appl. Polym. Mater., 2019, vol. 1, p. 83. https://doi.org/10.1021/acsapm.8b00061
Garreau, S., Duvail, J.L., and Louarn, G., Spectroelectrochemical studies of poly(3,4-ethylenedioxythiophene) in aqueous medium, Synth. Met., 2001, vol. 125, p. 325.https://doi.org/10.1016/S0379-6779(01)00397-6
Lukyanov, D.A., Vereshchagin, A.A., Soloviova, A.V., Grigorova, O.V., Vlasov, P.S., and Levin, O.V., Sulfonated Polycatechol Immobilized in a Conductive Polymer for Enhanced Energy Storage, ACS Appl. Energy Mater., 2021, vol. 4, p. 5070.https://doi.org/10.1021/acsaem.1c00639
Gribkova, O.L., Iakobson, O.D., Nekrasov, A.A., Cabanova, V.A., Tverskoy, V.A., Tameev, A.R., and Vannikov, A.V., Ultraviolet-Visible-Near Infrared and Raman spectroelectrochemistry of poly(3,4-ethylenedioxythiophene) complexes with sulfonated polyelectrolytes. The role of inter- and intra-molecular interactions in polyelectrolyte, Electrochim. Acta, 2016, vol. 222, p. 409.https://doi.org/10.1016/j.electacta.2016.10.193
Lokesh, K.S. and Adriaens, A., Electropolymerization of palladium tetraaminephthalocyanine: Characterization and supercapacitance behavior, Dye. Pigment., 2015, vol. 112, p. 192.https://doi.org/10.1016/j.dyepig.2014.06.034
Aroca, R., Dilella, D.P., and Loutfy, R.O., Raman spectra of solid films-I. Metal-free phthalocyanine, J. Phys. Chem. Solids, 1982, vol. 43, p. 707.https://doi.org/10.1016/0022-3697(82)90235-9
Peintler-Kriván, E., Tóth, P.S., and Visy, C., Combination of in situ UV-Vis-NIR spectro-electrochemical and a.c. impedance measurements: A new, effective technique for studying the redox transformation of conducting electroactive materials, Electrochem. commun., 2009, vol. 11, p. 1947.https://doi.org/10.1016/j.elecom.2009.08.025
Alpatova, N.M., Rotenberg, Z.A., Ovsyannikova, E.V., Topolev, V.V., Grosheva, M.Y., Kirchmeyer, S., and Jonas, F., Poly(3,4-ethylenedioxythiophene) heterogeneity: A differential cyclic voltabsorptometry study, Russ. J. Electrochem., 2004, vol. 40, p. 917.https://doi.org/10.1023/B:RUEL.0000041358.46069.d5
Yamato, H., Kai, K.I., Ohwa, M., Wernet, W., and Matsumura, M., Mechanical, electrochemical and optical properties of poly(3,4-ethylenedioxythiophene)/sulfated poly(β-hydroxyethers) composite films, Electrochim. Acta, 1997, vol. 42, p. 2517.https://doi.org/10.1016/S0013-4686(96)00442-2
ACKNOWLEDGMENTS
The present work is dedicated to the memory of Oleg Aleksadrovich Petrii, the outstanding researcher, teacher, and cordial interlocutor who exerted invaluable influence on the research biography of the authors (О.L. Gribkova and А.А. Nekrasov).
The electron absorption spectra in UV-, visible, and near IR-regions, Raman-spectra, and atomic-force microscopy images of the film surface were registered with the equipment of CKP FMI IPCE RAS.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-29-08048_mk, and the Ministry of Education and Science RF (study of sensor properties of developed composites).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
A tribute to outstanding electrochemist Oleg Aleksandrovich Petrii (1937–2021).
Rights and permissions
About this article
Cite this article
Gribkova, O.L., Kabanova, V.A., Yagodin, A.V. et al. Water-Soluble Phthalocyanine with Ionogenic Groups as a Molecular Template for Electropolymerization of 3,4-Ethylenedioxythiophene. Russ J Electrochem 58, 957–967 (2022). https://doi.org/10.1134/S1023193522110076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522110076