Abstract
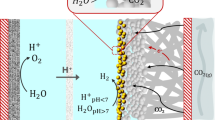
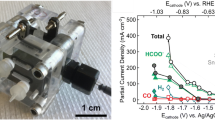
Carbon dioxide, as a greenhouse gas, is one of the most significant contributors to climate changes on the planet. The CO2 electrocatalytic reduction to value-added products is the key to the achieving of practical renewable energy conversion and storage, as well as green chemical production based on the CO2 and H2O. Sn- and Bi-based electrocatalysts are considered being the most promising for the electrolytic reduction of CO2 to formic acid, which has a wide range of applications, a pure hydrogen carrier, in particular. Due to the low solubility of carbon dioxide in aqueous solutions, the most promising high-rate and selective electrodes for its reduction are gas-diffusion electrodes, which make it possible to overcome restrictions on the mass transfer of the substrate (CO2) to a highly developed three-phase contact surface. In this review, we have systematized the most significant practical results obtained over the 2019–2021 period by various research groups in the electrocatalytic reduction of carbon dioxide to formic acid in aqueous electrolyte solutions at gas-diffusion electrodes based on Sn and Bi and their compounds.
Similar content being viewed by others
REFERENCES
Lim, R.J., Xie, M., Sk, M.A., Lee, J.M., Fisher, A., Wang, X., and Lim, K. H., A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts, Catal. Today, 2014, vol. 233, p. 169.
Liu, H., Zhu, Y., Ma, J., Zhang, Z., and Hu, W., Recent Advances in Atomic—Level Engineering of Nanostructured Catalysts for Electrochemical CO2 Reduction, Advanced Functional Mater., 2020, vol. 30, p. 1910534.
Du, D., Lan, R., Humphreys, J., and Tao, S., Progress in inorganic cathode catalysts for electrochemical conversion of carbon dioxide into formate or formic acid, J. Appl. Electrochem., 2017, vol. 47, p. 661.
Carbon Dioxide as Chemical Feedstosk, Aresta, M., Ed., Weinheim: Wiley–VCH, 2010.
Hori, Y., Electrochemical CO2 reduction on metal electrodes, In Modem Aspects of Electrochemistry, Ed. Vayenas, C.G., White, R.E., and Gamboa-Aldeco, M.E., vol. 42. N.Y.: Springer, 2008, p. 89–189.
Malkhandi, S. and Siang, Y., B., Electrochemical conversion of carbon dioxide to high value chemicals using gas diffusion electrodes, Curr. Opinion in Chem. Eng., 2019, vol. 26, p. 112.
Anastas, P.T. and Warner, J.C., Green Chemistry: Theory and practice, London: Oxford University Press, 1998, 144 p.
Li, L., Huang, Y., and Li, Y., Carbonaceous materials for electrochemical CO2 reduction, Energy Chem., 2020, vol. 2, p. 100024.
Weekes, D.M., Salvatore, D.A., Reyes, A., Huang A., and Berlinguette, C.P., Electrolytic CO2 reduction in a flow cell, Acc. Chem. Res., 2018, vol. 51, p. 910.
Burdyny, T. and Smith, W.A., CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially—relevant conditions, Energy Environ. Sci., 2019, vol. 12, p. 1442.
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T.F., and Weber, A.Z., Gas-diffusion electrodes for carbon dioxide reduction : a new paradigm, ACS Energy Lett., 2019, vol. 4, p. 317.
Kornienko, V.L., Kolyagin, G.A., Kornienko, G.V., Parfenov, V.A., and Ponomarenko, I.V., Electrosynthesis of H2O2 from O2 in Gas Diffusion Electrodes Based on Mesostructured Carbon CMK-3, Russ. J. Electrochem., 2018, vol. 54, p. 192.
Chatterjee, S., Dutta, I., Lum, Y., Lai, Z., and Huang, K.-W., Enanling storage and utilization of Low-carbon electricity: power to formic acid, Energy Environmental Sci., 2021, vol. 14, p. 1194.
Irtem, E., Andreu, T., Parra, A., Hernander-Alonso, M.D., Garcia-Rodriguez, S., Riesco-Garcia, J.M., Penelas-Perez, G., and Morante, J.R., Low-energy formate production from CO2 electroreduction using electrodeposited tin on GDE, J. Mater. Chem. A., 2016, vol. 4, p. 13582.
Del Castillo, A., Alvarez-Guerra, M., Solla-Gullon, J., Saez, A., Montiel, V., and Irabien, A., Sn nanoparticles on gas diffusion electrodes: synthesis, charactezation and use for continuous CO2 electroreduction to formate, J. Util. CO 2, 2017, vol. 18, p. 222.
Kopljar, D., Wagner, N., and Klemm, E., Transferring electrochemical CO2 reduction from semi-batch into continuous operation mode using gas diffusion electrodes, Chem. Eng. Technol., 2016, vol. 39, p. 2042.
Petrii, O.A., The progress in understanding the mechanisms of methanol and formic acid electrooxidation on platinum metals (a review), Russ. J. Electrochem., 2019, vol. 55, p. 1.
Finn, C., Schnittger, S., Yellowlees, L.J., and Love, J.B., Molecular approaches to the electrochemical reduction of carbon dioxide, Chem. Commun., 2012, vol. 48, p. 1392.
Agarw, A.S., Zhai, Y., Hill, D., and Sridhar, N., The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility, Chem. Sus. Chem., 2011, vol. 4, p. 1301.
Subin, P., Wijaya, D.T., Jonggeol, D.T., Na, J., and Lee, C.W., Towards the Lange-Scale Electrochemical Reduction of Carbon Dioxide, Catalysts, 2021, vol. 11, p. 253.
Tan, X., Yu, C., Ren, Y., Song, C., Li,W., and Qiu, J., Recent advances in innovative strategies the CO2 electroreduction reaction, Energy Environmental Sci., 2021, vol. 14, p. 765.
Shaima, A. Al-Tamreh, Mohamed, H. Ibrahim, Muftah, H. El – Naas, Jan, Vaes, Deepak, Pant, Abdelbaki, Benamor, and Abdulkaren, Amhamed, Electroreduction of Carbon Dioxide into Formate: A Comprehensive Review, ChemElectroChem., 2021,vol. 8, p. 3207.
Gao, D., Wei, P., Li, H., Lim, L., Wang G., and Bao, X., Designing Electrolyzers for Electrocatalytic CO2 Reduction, Acta Phys.-Chim. Sin., 2021, vol. 37, p. 2009021.
Bienen, F., Lowe, A., Hildebrant, J., Hertle, S., Schonfogel, D., Kopljar, D., Wagner, N., Klemm, E., and Fridrich, K.A., Degration study on tin- and bismuth-based gas-diffusion electrodes during electrochemical CO2 reduction in highly alkaline media, J. Energy Chem., 2021, vol. 62, p. 367.
Rabiee, H., Ge, L., Zhang, X., Hu, Shihu., Smart, S., Zhu, Z., Wang, H., and Yuan, Z., Stand – Alone asymmetric hollow fiber gas-diffusion electrodes with distinguished brobze phases for high-efficiency CO2 electrochemical reduction, Appl. Catal. B: Environmental, 2021, vol. 298, p. 120538.
An, X., Li, S., Hao, X., Xie, Z., Du, X., Wang, Z., Hao, X., Abudula, A., and Guan, G., Common strategies for improving the perfomances of tin and bismuth-based catalysts in the electrocatalytic reduction of CO2 to formic acid formate, Renewable and Sustainable Energy Rev., 2021, vol. 143, p. 110952.
Rablee, H., Ge, L., Zhang, X., Hu, S., Li, M., and Yuan, Z., Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value—added products: a review, Energy Enviro., Sci., 2021, vol. 14, p. 1959.
Grigioni, Ivan, Laxmi, Kishore Sagar, Yuguang, C., Li, Geonhni, Lee, Yu, Yan, Koen, Bertens, Rui Kai, Miao, Xue, Wang, Jehad, Abed, Da Hye, Wong, F., Pelayo, Garcia de Arquer, Alexander, H., Ip David, Sinton, and Edvard, H. Sargent, CO2 Electroreduction to Formate at a Partial Current Density of 930 mA cm–2 with Inp Colloidal Quantum Dot Derived Catalysts, ACS Energy Lett., 2021, vol. 6, p. 79.
Lowe, A., Schmidt, M., Bienen, F., Kopljar, D., Wagner, N., and Klemm, E., Optimizing reaction and gas diffusion electrodes applied in CO2 reduction reactiom to formate to reach current densities up to 1.8 A cm–2, ACS Sustainable Chem. Engineering, 2021, vol. 9, p. 4213.
Byeong-Uk, Choi, Ying Chuan, Tan, Hakhyeon, Song, Kelvin, Berm Lee, and Jihun, Oh, System Design Considerations for Enhancing for Electroreduction of Formate from Simulated Flue Gas, ACS Sustainable Chem. Eng., 2021, vol. 9, p. 2348.
Duarte, M., Daems, N., Hereijgers, J., Arenas-Esteban, D., Bals, S., and Breugelmans, T., Enhanced CO2 electroreduction with metal–nitrogen-doped carbons in a continuous flow reactor, J. CO 2 Utilization, 2021, vol. 50, p. 101583.
Prangan, D., Dibyajyoti, VSK Ya., and Mihir Kumar, P., Progress in the electrochemical reduction of CO2 to formic acid: A review on current trends and future prospects, J. Envirenmental Chem. Engineering, 2021, vol. 9, p. 106394.
Etienne, B. and Marc, R., Molecular Electrochemical Reduction of CO2 beyond two Electrons, Trends Chem., 2021, vol. 3, p. 359.
Senocrate, A. and Battaglia, C., Electrochemical CO2 reduction at room temperature status and perspectives, J. Energy Storage, 2021, vol. 36, p. 102373.
Guan, Y., Liu, M., Rao, X., Liu, Yu., and Zhang, J., Electrochemical reduction of carbon dioxide (CO2): bismuth-based electrocatalysts, J. Mater. Chem. A, 2021, vol. 9, p. 13770.
Zhang, S., Chen, C., Yu, H., and Li, F., Materials and System design for direct electrochemical CO2 conversion in capture media, J. Mater. Chem. A, 2021, vol. 9, p. 18785.
Cheng, F., Zhang, X., Mu, K., Jio, M., Wang, Z., Limpachanangkul, P., Chalermsinwan, B., Gao, Y., Li, Y., Chen, Z., and Lin, L., Recent Progress of Sn-Based Derivative catalysts for Electrochemical Reduction of CO2, Energy Technol., 2021, vol. 9, p. 2000799.
Wang, Q., Cai, Chao, Dai, M., Fu, J., Zhang, X., Li, H., Zhang, H., Chen, K., Lin, Y., Li, H., Hu, J., Miyauchi, M., and Liu, M., Recent Advances in Strategies for improving the Perfomance of CO2 Reduction Reaction on Single Atom Catalysts, Small Science, 2021, vol. 1, p. 2000028.
Thijs, B., Ronge, J., and Martens, J.A., Selective electrochemical reduction of CO2 to formic acid in a gas phase reactor with by-product recirculation, Sustainable Energy and Fuels, 2021, vol. 5, p. 1867.
Osetrova, N.V., Zaharyan, A.V., Vasilev, Yu.B., and Bagotskij, V.S., Electrochemical reduction of carbon dioxide, In: Elektrosintes monomerov (Advanced Electrochemical of Organic Compounds) (in Russian), Moscow: Nauka, 1980, p. 220–243.
Kornienko, V.L., Kolyagin, G.A., and Saltykov, Yu.V., Electrosynthesis in Waterproofed Electrodes (in Russian), Novosibirsk: Sib. Branch, Russ. Akad. Nauk, 2011. (http://www.rfbr.ru/rffi/ru/books/o_1781580#1)
Qian, Y., Liu, Y., Tang, H., and Lin, B.-L., Highly efficient electroreduction of CO2 to formate by nanorod@2D nanosheets SnO, J. CO 2 Utilization, 2020, vol. 42, p. 101287.
Sakaishi, K., Oaki, Y., Uchiyama, H., Hosono, E., Zhou, H., and Imai, H., Aqueus solution synthesis of SnO nanostructures with tuned optical absorption behavior and photoelectrochemical properties through morphological evolution, Nanoscale, 2020, vol. 2, p. 2424.
Wang, Q., Wu, Y., Zhu, C., Xiong, R., Deng, Y., Wang, X., Wu, C., and Yu, H., Sn nanoparticles deposited onto a gas diffusion layer via impregnation-electroreduction for enhanced CO2 electroreduction to formate, Electrochim. Acta, 2021, vol. 369, p. 137662.
Lim, J., Kang, P.W., Jeon, S.S., and Lee, H., Electrochemically deposited Sn catalysts with dense tips on a gas diffusion electrode for electrochemical CO2 reduction, J. Mater. Chem. A, 2020, vol. 8, p. 9032.
Li, M., Idros, M.N., Wu, Y., Garg, S., Gao, S., Lin, R., Rabiee, H., Li, Z., Ge, L., Rufford, T.E., Zhu, Z., Lid, L., and Wang, G., Unveiling the effects of dimensionality of tin oxide-derived catalysts on CO2 reduction by using gas-diffusion electrodes, React. Chem. Eng., 2021, vol. 6, p. 345.
Löwe, A., Rieg, C., Hierlemann, T., Salas, N., Kopljar, D., Wagner, N., and Klemm, E., Influence of Temperature on the Performance of Gas Diffusion Electrodes in the CO2 Reduction Reaction, ChemElectroChem, 2019, vol. 6, p. 4497.
Chen, Y., Vise, A., Klein, W.E., Cetinbas, F.C., Kariuki, N.N., Myers, D.J., Smith, W.A., Deutsch, T.G., and Neyerlin, K.C., A robust, scalable platform for the electrochemical conversion of CO2 to formate; identifying pathways to higher energy efficiencies, ACS Energy Lett., 2020, vol. 5, p. 1825.
Sen, S., Brown, S.M., Leonard, McL., and Brushett, F.R., Electroreduction of carbon dioxide to formate at high current densities using tin and tin oxide gas diffusion electrodes, J. Appl. Electrochem., 2019, vol. 49, p. 917.
Wang, J., Zou, J., Hu, X., Ning, S., Wang, X., Kang, X., and Chen, S., Heterostructured intermetallic CuSn catalysts: high performance towards the electrochemical reduction of CO2 to formate, J. Mater. Chem. A, 2019, vol. 7, p. 27514.
Bienen, F., Kopljar, D., Lowe, A, Aßmann, P., Stoll, M., Roßner, P., Wagner, N., Friedrich, A., and Klemm, E., Utilizing Formate as an Energy Carrier by Coupling CO2 Electrolysis with Fuel Cell Devices, Chem. Ing. Tech., 2019, vol. 91, p. 872.
Xiang, H., Miller, H.A., Bellini, M., Christensen, H., Scott, K., Rasul, S., and Yu, E.H., Production of formate by CO2 electrochemical reduction and its application in energy storage, Sustainable Energy and Fuels, 2020, vol. 4, p. 277.
Ma, W., Bu, J., Liu, Z., Yan, C., Yao, Y., Chang, N., Zhang, H., Wang, T., and Zhang, J., Monoclinic Scheelite Bismuth Vanadate Derived Bismuthene Nanosheets with Rapid Kinetics for Electrochemically Reducing Carbon Dioxide to Formate, Adv. Funct. Mater., 2021, vol. 31, p. 2006704.
Fan, T., Ma, W., Xie, M., Liu, H., Zhang, Yang, S., Huang, P., Dong, Y., Chen, Z., and Yi, X., Achieving high current density for electrocatalytic reduction of CO2 to formate on bismuth-based catalysts, Cell Reports Phys. Sci., 2021, vol. 2, p. 100353.
Yang, J., Wang, X., Qu, Y., Wang, X., Huo, H., Fan, Q., Wang, J., Yang, L.-M., and Wu, Y., Bi-dased metal-organic framework Derived Leafy Bismuth Nanosheets for Carbon Dioxide Electroreduction, Adv. Energy Mater., 2020, vol. 10, p. 2001709.
Gong, Q., Ding, P., Xu, M., Zhu, X., Wang, M., Deng, J., Ma, Q., Han, N., Zhu, Y., Lu, J., Feng, Z., Li, Y., Zhou, W., and Li, Y., Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction, Nature Commun., 2019, vol. 10, p. 2807.
Wang, Q., Zhu, C., Wu, C., and Yu, H., Direct synthesis of bismuth nanosheets on a gas diffusion layer as a high-performance cathode for a coupled electrochemical system capable of electroreduction of CO2 to formate with simultaneous degradation of organic pollutants, Electrochim. Acta, 2019, vol. 319, p. 138.
Xing, Z., Hu, X., and Feng, X., Tuning the Microenvironment in Gas-Diffusion Electrodes Enables High-Rate CO2 Electrolysis to Formate, ACS Energy Lett, 2021, vol. 6, p. 1694.
Diaz-Sainz, G., Alvarez-Guerra, M., Solla-Gullon, J., Garcia-Cruz, L., Montiel, V., and Irabien, A., CO2 electroreduction to formate: Continuous single-pass operation in a filter-press reactor at high current densities using Bi gas diffusion electrodes, J. CO 2 Utilization, 2019, vol. 34, p. 12.
Rabiee, H., Ge, L., Zhang, X., Hu, S., Li, M., Smart, S., Zhu, Z., and Yuan, Z., Shape-tuned electrodeposition of bismuth-based nanosheets on flow-through hollow fiber gas diffusion electrode for high-efficiency CO2 reduction to formate, Appl. Catal. B: Environmental, 2021, vol. 286, p. 119945.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
A tribute to outstanding electrochemist Oleg Aleksandrovich Petrii (1937–2021).
Rights and permissions
About this article
Cite this article
Kornienko, V.L., Kolyagin, G.A. & Taran, O.P. Electrocatalytic Reduction of Carbon Dioxide to Formic Acid on Sn- and Bi-Based Gas-Diffusion Electrodes in Aqueous Media (a Review). Russ J Electrochem 58, 647–657 (2022). https://doi.org/10.1134/S1023193522080079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522080079