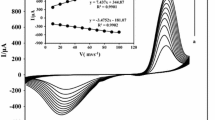
Abstract
In this work Cu modified carbon paste electrode was prepared using Cu2+ ions reduction according to electrodeposition method. Then using galvanic replacement reaction, one layer of Ag was deposited on the surface of electrode. The electrochemical behavior of the modified carbon paste electrode was studied by electrochemical methods such as cyclic voltammetry and chronoamperometry. This Ag/Cu modified carbon paste electrode shows good activity for electrocatalytic oxidation of ethylene glychol. The parameters which are effective on response of modified electrode to oxidation of ethylene glychol, such as applied potential and duration of potential applying for electrodeposition of Cu, duration of galvanic replacement reaction in AgNO3 solution and the kind of acidic solution for electrodeposition of Cu were optimized. The morphology of modified carbon paste electrodes was investigated by field emission scanning electron microscopy and Energy-dispersive X-ray spectroscopy. Stability of prepared electrode as an anode for oxidation of ethylene glychol was studied. Finaly current density of ethylene glychol oxidation at the surface of proposed electrode was compared with other electrodes reported in literatures. Results show that proposed electrode, owing to having advantages such as appropriate current density, long time stability, not poisoning with ethylene glychol oxidation products, easy and low cost preparation method, can be a good choice as anode of fuel cells.









Similar content being viewed by others
REFERENCES
Sartorida Silva, F. and Miguelde Souza, T., Novel materials for solid oxide fuel cell technologies: s literature review, Int. J. Hydrogen Energy, 2017, vol. 42, p. 26020.
Wang, G., Yu, Y., Liu, H., Gong, C., Wen, S., Wang, X., and Tu, Z., Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: a review, Fuel Process. Technol., 2018, vol. 179, p. 203.
Lu, X., Wu, Y., Lian, J., Zhang, Y., Chen, C., Wang, P., and Meng, L., Energy management of hybrid electric vehicles: A review of energy optimization of fuel cell hybrid power system based on genetic algorithm, Energy Convers. Manag., 2020, vol. 205, p. 112474.
Olabi, A.G., Wilberforce, T., and Abdelkareem, M.A., Fuel cell application in the automotive industry and future perspective, Energy, 2021, vol. 214, p. 118955.
Serov, A. and Kwak, C., Recent achievements in direct ethylene glycol fuel cells, Appl. Catal. B: Environ., 2010, vol. 97, p. 1.
An, L. and Chen, R., Recent progress in alkaline direct ethylene glycol fuel cells for sustainable energy production, J. Power Sources, 2016, vol. 329, p. 484.
Livshits, V. and Peled, E., Progress in the development of a high-power, direct ethylene glycol fuel cell, J. Power Sources, 2006, vol. 161, p. 1187.
Yue, H., Zhao, Y., Ma, X., and Gong, J., Ethylene glycol: properties, synthesis, and applications, Chem. Soc. Rev., 2012, vol. 41, p. 4218.
Kim, H.J., Choi, S.M., Green, S., Tompsett, G.A., Lee, S.H., Huber, G.W., and Kim, W.B., Highly active and stable PtRuSn/C catalyst for electrooxidations of ethylene glycol and glycerol, Appl. Catal. B: Environ., 2011, vol. 101, p. 366.
Livshits, V., Philosoph, M., and Peled, E., Direct ethylene glycol fuel-cell stack-study of oxidation intermediate products, J. Power Sources, 2008, vol. 178, p. 687.
Gao, H., Zhai, C., Yuan, C., Liu, Z.Q., and Zhu, M., Snowflake-like Cu2S as visible-light-carrier for boosting Pd electrocatalytic ethylene glycol oxidation under visible light irradiation, Electrochim. Acta, 2020, vol. 330, p. 135214.
Lin, Q., Wei, Y., Liu, W., Yu, Y., and Hu, J., Electrocatalytic oxidation of ethylene glycol and glycerol on nickel ion implanted-modified indium tin oxide electrode, Int. J. Hydrogen Energy, 2017, vol. 42, p. 1403.
Jing Lv, J., Shan Li, S., Zheng, J.N., Wang, A.J., Chen, J.R., and Feng, J.J., Facile synthesis of reduced graphene oxide supported PtAg nanoflowers and their enhanced electrocatalytic activity, Int. J. Hydrogen Energy, 2014, vol. 39, p. 3211.
Krittayavathananon, A. and Sawangphruk, M., Electrocatalytic oxidation of ethylene glycol on palladium coated on 3D reduced graphene oxide aerogel paper in alkali media: effects of carbon supports and hydrodynamic diffusion, Electrochim. Acta, 2016, vol. 212, p. 237.
Fashedemi, O.O. and Ozoemena, K.I., Comparative electrocatalytic oxidation of ethanol, ethylene glycol and glycerol in alkaline medium at Pd-decorated FeCo@Fe/C coreshell nanocatalysts, Electrochim. Acta, 2014, vol. 10, p. 279.
Chen, S.S., Yang, Z.Z., Wang, A.J., Fang, K.M., and Feng, J.J., Facile synthesis of bimetallic gold palladium nanocrystals as effective and durable advanced catalysts for improved electrocatalytic performances of ethylene glycol and glycerol oxidation, J. Colloid Interface Sci., 2018, vol. 509, p. 7.
Su, W., Sun, R., Ren, W., Yao, Y., Fei, Z., Wang, H., et al., Graphene supported palladium phosphorus nanoparticles as a promising catalyst for ethylene glycol oxidation, Appl. Surf. Sci., 2019, vol. 91, p. 735.
Shi, Y.C., Feng, J.J., Lin, X.X., Zhang, L., Yuan, J., Zhang, Q.L., et al., One-step hydrothermal synthesis of three-dimensional nitrogen-doped reduced graphene oxide hydrogels anchored PtPd alloyed nanoparticles for ethylene glycol oxidation and hydrogen evolution reactions, Electrochim. Acta, 2019, vol. 293, p. 504.
Duan, J.J., Zheng, X.X. Niu, H.J., Feng, J.J., Zhang, Q.L., Huang, H., and Wang, A.J., Porous dendritic PtRuPd nanospheres with enhanced catalytic activity and durability for ethylene glycol oxidation and oxygen reduction reactions, J. Colloid. Interface. Sci., 2020, vol. 560, p. 467.
Arul, P. and Abraham John, S., Electrodeposition of CuO from Cu-MOF on glassy carbon electrode: a non-enzymatic sensor for glucose, J. Electroanal. Chem., 2017, vol. 799, p. 61.
Raoof, J.B., Rashid-Nadimi, S., and Ojani, R., Parametric study on electrochemical deposition of hair shaped PtRu as methanol oxidation catalyst, Int. J. Hydrogen Energy, 2013, vol. 38, p. 16062.
Kumar, S., Pande, S., and Verma, P., Factor effecting electro-deposition process, Int. J. Curr. Eng. Technol., 2015, vol. 5, article 700.
Papaderakis, A., Mintsouli, I., Georgieva, J., and Sotiropoulos, S., Electrocatalysts prepared by galvanic replacement, Catalysts, 2017, vol. 7, p. 80.
Werbicki, J.J., Practical electroless and immersion plating, Plating, 1971, vol. 58, p. 763.
Wingenfeld, P., Advanced technology for selective plating of connectors, Met. Finish, 1994, vol. 92, p. 13.
Walsh, D.E., Milad, G., and Gudeczauskas, D., Final finish: printed circuit boards, Met. Finish, 2003, vol. 101, p. 25.
Bliznakov, S., Vukmirovic, M., and Adzic, R., Electrochemical atomic-level controlled syntheses of electrocatalysts for the oxygen reduction reaction, RSC Catal. Ser., 2015, vol. 22, p. 144.
Zhang, J., Vukmirovic, M.B., Xu, Y., Mavrikakis, M., and Adzic, R.R., Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates, Angew. Chem. Int. Ed., 2005, vol. 44, p. 2132.
Zhang, J., Vukmirovic, M.B., Sasaki, K., Nilekar, A.U., Mavrikakis, M., and Adzic, R.R., Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics, J. Am. Chem. Soc., 2005, vol. 127, p. 12480.
Shao, M., Sasaki, K., Marinkovic, N.S., Zhang, L., and Adzic, R.R., Synthesis and characterization of platinum monolayer oxygen–reduction electrocatalysts with Co–Pd core–shell nanoparticle supports, Electrochem. Commun., 2007, vol. 9, p. 2848.
Mintsouli, I., Georgieva, J., Valova, E., Armyanov, S., Kakaroglou, A., Hubin, A., Steenhaut, O., Dille, J., Papaderakis, A., Kokkinidis, G., et al., Pt–Ni carbon-supported catalysts for methanol oxidation prepared by Ni electroless deposition and its galvanic replacement by Pt, J. Solid State Electrochem., 2013, vol. 17, p. 435.
Zheng, F., Luk, S.Y., Kwong, T.L., and Yung, K.F., Synthesis of hollow PtAg alloy nanospheres with excellent electrocatalytic performances towards methanol and formic acid oxidations, RSC Adv., 2016, vol. 6, p. 44902.
Li, C., Su, Y., Lv, X., Shi, H., Yang, X., and Wang, Y., Enhanced ethanol electrooxidation of hollow Pd nanospheres prepared by galvanic exchange reactions, Mater. Lett., 2012, vol. 69, p. 92.
Rezaei, B., Mokhtarianpour, M., and Ensafi, A.A., Fabricated of bimetallic Pd/Pt nanostructure deposited on copper nanofoam substrate by galvanic replacement as an effective electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2015, vol. 40, p. 6754.
Elbert, K., Hu, J., Ma, Z., Zhang, Y., Chen, G., An, W., Liu, P., Isaacs, H.S., Adzic, R.R., and Wang, J.X., Elucidating hydrogen oxidation/evolution kinetics in base and acid by enhanced activities at the optimized Pt shell thickness on the Ru core, A.C.S. Catal., 2015, vol. 5, p. 6764.
Papaderakis, A., Pliatsikas, N., Prochaska, C., Vourlias, G., Patsalas, P., Tsiplakides, D., Balomenou, S., and Sotiropoulos, S., Oxygen evolution at IrO2 shell-Ir–Ni core electrodes prepared by galvanic replacement, J. Phys. Chem. C, 2016, vol. 120, p. 19995.
Hosseini, M.G., Abdolmaleki, M., and Nasirpouri, F., Investigation of the porous nanostructured Cu/Ni/AuNi electrode for sodium borohydride electrooxidation, Electrochim. Acta, 2013, vol. 114, p. 215.
Vanrenterghem, B., Papaderakis, A., Sotiropoulos, S., Tsiplakides, D., Balomenou, S., Bals, S., and Breugelmans, T., The reduction of benzylbromide at Ag–Ni deposits prepared by galvanic replacement, Electrochim. Acta, 2016, vol. 196, p. 756.
Ahmadi Diva, A., Fathi, Sh., and Chekin, F., Determination of fluvoxamine in real samples using carbon paste electrode modified by electrodeposition of nickel, J. Anal. Chem., 2019, vol. 74, p. 809.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, 2nd ed., New York: Wiley, 2001.
Kehoe, D.K. Romeral, L., Lundy, R., Morris, M.A., Lyons, M.G., and Gun’ko, Y.K., One Dimensional AuAg nanostructures as anodic catalysts in the ethylene glycol oxidation, Nanomaterials, 2020, vol. 10, p. 719.
Rigsby, M.A., Spurlin, T.A., and Reid, J.D., The multi-functional role of boric acid in cobalt electrodeposition and superfill, J. Electrochem. Soc., 2020, vol. 167, p. 112507.
ACKNOWLEDGMENTS
The authors are sincerely thankful for the research facilities provided by the Ayatollah Amoli Branch of the Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohammad Mirzaei, Ghadi, A. & Fathi, S. Preparation of a Modified Electrode Using Electrodeposition of Cu Followed by Galvanic Replacement of Ag: Application for Electrocatalytic Oxidation of Ethylen Glychol. Russ J Electrochem 58, 192–201 (2022). https://doi.org/10.1134/S1023193522030089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522030089