Abstract
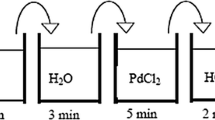
The equations for the concentration of metallic ions in the electroless plating solution have been derived. They are used to predict the effect of plating para- meters(i.e. pH, the concentration of ethylenediaminetetraacetic acid disodium salt (Na2EDTA) and plating temperature) on membrane composition in Pd‒Ag electroless plating theoretically. The experiments have shown that more palladium was deposited as the concentration of Na2EDTA and pH was increased, while the temperature was decreased.






Similar content being viewed by others
REFERENCES
Lin, Y.-M. and Rei, M.-H., Separation of hydrogen from the gas mixture out of catalytic reformer by using supported palladium membrane, Sep. Purif. Technol., 2001, vol. 25, p. 87.
Bruni, G., Rizzello, C., Santucci, A., Alique, D., Incelli, M., and Tosti, S., On the energy efficiency of hydrogen production processes via steam reforming using membrane reactors, Int. J. Hydrogen Energy, 2019, vol. 44, p. 988.
Dittmeyer, R., Höllein, V., and Daub, K., Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium, J. Mol. Catal. A: Chem., 2001, vol. 173, p. 135.
Zhao, C., Xu, H., and Goldbach, A., Duplex Pd/ceramic/Pd composite membrane for sweep gas-enhanced CO2 capture, J. Membr. Sci., 2018, vol. 563, p. 388.
Li, A., Liang, W., and Hughes, R., Fabrication of dense palladium composite membranes for hydrogen separation, Catal. Today, 2000, vol. 56, p. 45.
Ryi, S.-K., Xu, N., Li, A., Lim, C.J., and Grace, J.R., Electroless Pd membrane deposition on alumina modified porous Hastelloy substrate with EDTA-free bath, Int. J. Hydrogen Energy, 2010, vol. 35, p. 2328.
Chen, W., Hu, X., Wang, R., and Huang, Y., On the assembling of Pd/ceramic composite membranes for hydrogen separation, Sep. Purif. Technol., 2010, vol. 72, p. 92.
Knapton, A.G., Palladium alloys for hydrogen diffusion membranes, Platinum Met. Rev., 1977, vol. 21, p. 44.
Li, H., Pieterse, J.A.Z., Dijkstra, J.W., Haije, W.G., Xu, H.Y., and Bao, C., Performance test of a bench-scale multi-tubular membrane reformer, J. Membr. Sci., 2011, vol. 373, p. 43.
Maneerung, T., Hidajat, K., and Kawi, S., Ultra-thin (<1 μm) internally-coated Pd–Ag alloyhollow fiber membrane with superior thermal stability and durability for high temperature H2 separation, J. Membr. Sci., 2014, vol. 45, p. 127.
Ghasemzadeh, K., Khosravi, M., Sadati Tilebon, S.M., Ghaeinejad-Meybodi, A., and Basile, A., Theoretical evaluation of Pd Ag membrane reactor performance during biomass steam gasification for hydrogen production using CFD method, Int. J. Hydrogen Energy, 2018, vol. 43, p. 117.
Melendez, J., Fernandez, E., Gallucci, F., van Sint Annaland, M., Arias, P.L., and Tanaka, D.A.P., Preparation and characterization of ceramic supported ultra-thin (~1 μm) Pd–Ag membranes, J. Membr. Sci., 2017, vol. 528, p. 12.
Vásquez Castillo, J.M., Sato, T., and Itoh, N., Effect of temperature and pressure on hydrogen production from steam reforming of biogas with Pd–Ag membrane reactor, Int. J. Hydrogen Energy, 2015, vol. 40, p. 3582.
Alique, D., Martinez-Diaz, D., Sanz, R., and Calles, J.A., Review of supported Pd-based membranes preparation by electroless plating for ultra-pure hydrogen production, Membrane, 2018, vol. 8, p. 1.
Fernandez, E., Helmi, A., Coenen, K., Melendez, J., Viviente, J.L., Tanaka, D.A.P., Annaland, M.S., and Gallucci, F., Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases, Int. J. Hydrogen Energy, 2015, vol. 40, p. 3506.
Checchetto, R., Bazzanella, N., Patton, B., and Miotello, A., Palladium membranes prepared by r.f. magnetron sputtering for hydrogen purification, Surf. Coat. Technol., 2004, vol. 177, p. 73.
Huang, L., Chen, C.S., He, Z.D., Peng, D.K., and Meng, G.Y., Palladium membranes supported on porous ceramics prepared by chemical vapor deposition, Thin Solid Films, 1997, vol. 302, p. 98.
Sumrunronnasak, S., Tantayanon, S., and Kiatgamolchai, S., Influence of layer compositions and annealing conditions on complete formation of ternary PdAgCu alloys prepared by sequential electroless and electroplating methods, Mater. Chem. Phys., 2017, vol. 185, p. 98.
Pujari, M., Agarwal, A., Uppaluri, R., and Verma, A., Role of electroless nickel diffusion barrier on the combinatorial plating characteristics of dense Pd/Ni/PSS composite membranes, Appl. Surf. Sci., 2014, vol. 305, p. 658.
Zhang, D., Zhou, S., Fan, Y., Xu, N., and He, Y., Preparation of dense Pd composite membranes on porous Ti–Al alloy supports by electroless plating, J. Membr. Sci., 2012, vols. 387–388, p. 24.
Martinez-Diaz, D., Sanz, R., Calles, J.A., and Alique, D., H2 permeation increase of electroless pore-plated Pd/PSS membranes with CeO2 intermediate barriers, Sep. Purif. Technol., 2019, vol. 216, p. 16.
Chotirach, M., Tantayanon, S., Tungasmita, S., and Kriausakul, K., Zr-based intermetallic diffusion barriers for stainless steel supported palladium membranes, J. Membr. Sci., 2012, vol. 405–406, p. 92.
Cheng, Y.S. and Yeung, K.L., Palladium–silver composite membranes by electroless plating technique, J. Membr. Sci., 1999, vol. 158, p. 127.
Bindra, P., Light, D., and Rath, D., Mechanisms of electroless metal plating: 1. Mixed potential theory and the interdependence of partial reactions, IBM J. RES. DEVELOP., 1984, vol. 28, p. 668.
Shu, J., Grandjean, B.P.A., Ghali, E., and Kaliaguine, S., Simultaneous deposition of Pd and Ag on porous stainless steel by electroless plating, J. Membr. Sci., 1993, vol. 77, p. 181.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors announce that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Chol-Man Pak, Han, UC., Kang, HJ. et al. Effect of Plating Parameters on Composition of Electroless Co-Deposited PdAg Membrane. Russ J Electrochem 57, 1207–1212 (2021). https://doi.org/10.1134/S1023193521110069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521110069