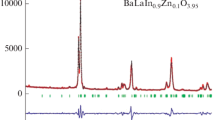
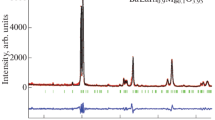
Abstract
The effect of donor doping Nb5+ → In3+ on the transport characteristics of the complex oxide BaLaInO4 with the Ruddlesden–Popper structure is analyzed. It is shown that the resulting phases are capable of dissociative absorption of water from the gas phase and manifestation of protonic conduction. It is found that the donor doping of BaLaInO4 leads to the increase in the oxygen-ionic conductivity.












Similar content being viewed by others
REFERENCES
Ruddlesden, S.N. and Popper, P., New compounds of the K2NiF4 type, Acta Crystallogr., 1957, vol. 10, p. 538.
Beznosikov, B.V. and Aleksandrov, K.S., Perovskite-like crystals of the Ruddlesden–Popper series, Crystallogr. Rep., 2000, vol. 45, p. 792.
Le Page, Y., Structural properties of Ba2RCu3O7 high-T c superconductors, Phys. Rev. B., 1987, vol. 36, p. 3517.
Cheong, S-W., Thompson, J.D., and Fisk, Z., Properties of La2CuO4 and related compounds, Phys. C., 1989, vol. 158, p. 109.
Moritomo, Y., Tomioka, Y., Asamitsu, A., and Tokura, Y., Magnetic and electronic properties in hole-doped manganese oxides with layered structures: La1 – xSr1 + xMnO4, Phys. Rev. B., 1995, vol. 51, p. 3297.
Hector, A.L., Knee, C.S., MacDonald, A.I., Price, D.J., and Weller, M.T., An unusual magnetic structure in Sr2FeO3F and magnetic structures of K2NiF4-type iron(III) oxides and oxide halides, including the cobalt substituted series Sr2Fe1 – xCoxO3Cl, J. Mater. Chem., 2005, vol. 15, p. 3093.
Sayers, R., Liu, J., Rustumji, B., and Skinner, S.J., Novel K2NiF4-type materials for solid oxide fuel cells: Compatibility with electrolytes in the intermediate temperature range, Fuel Cell, 2008, vol. 8, p. 338.
Montenegro-Hernandez, A., Vega-Castillo, J., Mogni, L., and Caneiro, A., Thermal stability of Ln2NiO4 + δ (Ln: La, Pr, Nd) and their chemical compatibility with YSZ and CGO solid electrolytes, Int. J. Hydrogen Energy, 2011, vol. 36, p. 15704.
Grimaud, A., Mauvy, F., Bassat, J.M., Fourcade, S., Marrony, M., and Grenier, J.C., Hydration and transport properties of the Pr2 – xSrxNiO4 + δ compounds as H+-SOFC cathodes, J. Mater. Chem., 2012, vol. 22, p. 16017.
Vibhu, V., Rougier, A., Nicollet, C., Flura, A., Fourcade, S., Penin, N., Grenier, J.C., and Bassat, J.M., Pr4Ni3O10 + δ: A new promising oxygen electrode material for solid oxide fuel cells, J. Power Sources, 2016, vol. 317, p. 184.
Yatoo, M.A., Du, Z., Zhao, H., Aguadero, A., and Skinner, S.J., La2Pr2Ni3O10 ± δ Ruddlesden–Popper phase as potential intermediate temperature-solid oxide fuel cell cathodes, Solid State Ionics, 2018, vol. 320, p. 148.
Mahato, N., Banerjee, A., Gupta, A., Omar, S., and Balani, K., Progress in material selection for solid oxide fuel cell technology: A review, Prog. Mater. Sci., 2015, vol. 72, p. 141.
Kan, W.H., Samson, A.J., and Thangadurai, V., Trends in electrode development for next generation solid oxide fuel cells, J. Mater. Chem. A., 2016, vol. 4, p. 17913.
Yesid Gómez, S. and Hotza, D., Current developments in reversible solid oxide fuel cells, Renewable Sustainable Energy Rev., 2016, vol. 61, p. 155.
da Silva, F.S. and de Souza, T.M., Novel materials for solid oxide fuel cell technologies: A literature review, Int. J. Hydrogen Energy, 2017, vol. 42, p. 26020.
Zhang, Y., Knibbe, R., Sunarso, J., Zhong, Y., Zhou, W., Shao, Z., and Zhu, Z., Recent progress on advanced materials for solid-oxide fuel cells operating below 500°C, Adv. Mater., 2017, vol. 29, p. 1700132.
Medvedev, D.A., Lyagaeva, J.G., Gorbova, E.V., Demin, A.K., and Tsiakaras, P., Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes, Prog. Mater. Sci., 2016, vol. 75, p. 38.
Danilov, N., Lyagaeva, J., Vdovin, G., and Medvedev, D., Multifactor performance analysis of reversible solid oxide cells based on proton-conducting electrolytes, Appl. Energy, 2019, vol. 237, p. 924.
Tarancon, A., Strategies for lowering solid oxide fuel cells operating temperature, Energies (Basel, Switz.), 2009, vol. 2, p. 1130.
Kochetova, N., Animitsa, I., and Medvedev, D., Recent activity in the development of proton-conducting oxides for high-temperature applications, RSC Adv., 2016, vol. 6, p. 73222.
Wachsman, E.D. and Lee, K.T., Lowering the temperature of solid oxide fuel cells, Science, 2011, vol. 334, p. 935.
Sood, K., Singh, K., and Pandey, O.P., Co-existence of cubic and orthorhombic phases in Ba-doped LaInO3 and their effect on conductivity, Phys. B., 2015, vol. 456, p. 250.
Byeon, D.-S., Jeong, S.-M., Hwang, K.-J., Yoon, M.-Y., Hwang, H.-J., Kim, S., and Lee, H.-L., Oxide ion diffusion in Ba-doped LaInO3 perovskite: A molecular dynamics study, J. Power Sources, 2013, vol. 222, p. 282.
Hwang, K.-J., Hwang, H.-J., Lee, M.-H., Jeong, S.-M., and Shin, T.-H., The effect of Co-doping at the A-site on the structure and oxide ion conductivity in (Ba0.5 – xSrx)La0.5InO3 – δ: A molecular dynamics study, Materials, 2019, vol. 12, p. 3739.
Schober, T., Friedrich, J., and Krug, F., Phase transformation in the oxygen and proton conductor Ba2In2O5 in humid atmospheres below 300°C, Solid State Ionics, 1997, vol. 99, p. 9.
Fisher, C.A.J. and Islam, M.S., Detect, protons and conductivity in brounmillerite–structured Ba2In2O5, Solid State Ionics, 1999, vol. 118, p. 355.
Kakinuma, K., Yamamura, H., and Haneda, H., Oxide-ion conductivity of the perovskite–type solid–solution system, (Ba1 – x – ySrxLay)2In2O5 + y , Solid State Ionics, 2002, vol. 154, p. 571.
Ta, T.Q., Tsuji, T., and Yamamura, Y., Thermal and electrical properties of Ba2In2O5 substituted for In site by rare earth elements, J. Alloys Compd., 2006, vol. 408, p. 253.
Jarry, A., Quarez, E., and Kravchyk, K., Rare earth effect on conductivity and stability properties of doped barium indates as potential proton-conducting fuel cell electrolytes, Solid State Ionics, 2012, vol. 216, p. 11.
Tarasova, N. and Animitsa, I., The influence of anionic heterovalent doping on transport properties and chemical stability of F-, Cl-doped brownmillerite Ba2In2O5, J. Alloys Compd., 2018, vol. 739, p. 353.
Tarasova, N. and Animitsa, I., Anionic doping (F–, Cl–) as the method for improving transport properties of proton-conducting perovskites based on Ba2CaNbO5.5, Solid State Ionics, 2018, vol. 317, p. 21.
Tarasova, N., Animitsa, I., Galisheva, A., and Korona, D., Incorporation and conduction of protons in Ca, Sr, Ba-doped BaLaInO4 with Ruddlesden-Popper structure, Materials, 2019, vol. 12, p. 1668.
Tarasova, N., Animitsa, I., Galisheva, A., and Prya-khina, V., Protonic transport in the new phases BaLaIn0.9M0.1O4.05 (M=Ti, Zr) with Ruddlesden–Popper structure, Solid State Sciences, 2020, vol. 101, p. 106121.
Tarasova, N., Animitsa, I., and Galisheva, A., Electrical properties of new protonic conductors Ba1 + xLa1 – xInO4 – 0.5x with Ruddlesden–Popper structure, J. Solid State Electrochem., 2020, vol. 24, p. 1497.
Tarasova, N., Galisheva, A., and Animitsa, I., Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping, Ionics, 2020, vol. 26, p. 5075.
Korona, D.V., Obrubova, A.V., Kozlyuk, A.O., and Animitsa, I.E., Hydration and proton transport in BaCaxLa1 – xInO4 – 0.5x (x = 0.1 and 0.2) phases with layered structure, Russ. J. Phys. Chem., 2018, vol. 92, p. 1727.
Titov, Yu.A., Belyavina, N.M., and Markiv, V.Ya., Synthesis and crystal structure of BaLaInO4 and SrLnInO4 (Ln–La, Pr), Rep. Nat. Acad. Sci. Ukraine, 2009, vol. 10, p. 160.
Troncoso, L., Alonso, J.A., Fernández-Díaz, M.T., and Aguadero, A., Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1 – xBxO4 + δ system (B = Zr, Ti), Solid State Ionics, 2015, vol. 82, p. 282.
Troncoso, L., Arce, M.D., Fernández-Díaz, M.T., Mogni, L.V., and Alonso, J.A., Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8 – xBaxInO4 + δ, New J. Chem., 2019, vol. 43, p. 6087.
Tarasova, N.A., Galisheva, A.O., Animitsa, I.E., and Korona, D.V., Hydration and the state of oxygen–hydrogen groups in the complex oxide BaLaIn0.9Nb0.1O4.1 with the Ruddlesden–Popper structure, Russ. J. Phys. Chem., 2020, vol. 94, p. 818.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Safonova
Based on the materials of the report at the 15th International Meeting “Fundamental Problems of Solid State Ionics,” Chernogolovka, 30.11.–07.12.2020.
Rights and permissions
About this article
Cite this article
Tarasova, N.A., Galisheva, A.O., Animitsa, I.E. et al. The Effect of Donor Doping on the Ionic (О2–, Н+) Transport in Novel Complex Oxides BaLaIn1 – xNbxO4 + x with the Ruddlesden–Popper Structure. Russ J Electrochem 57, 962–969 (2021). https://doi.org/10.1134/S1023193521080115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521080115