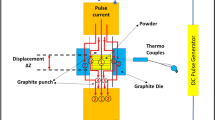
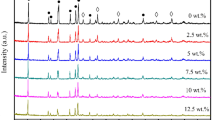
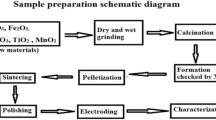
Abstract
Composite materials CaY2S4–ZrS2 and BaSm2S4–ZrS2 synthesized from oxide precursors by ceramic and citrate–nitrate methods, are certified with X-ray diffraction analysis and electron microprobe analysis with subsequent mapping. The use of citrate–nitrate conditioning of the BaSm2S4–ZrS2 composite samples leads to the product contamination, while ceramic conditioning allows obtaining heterogeneous system: solid solution of samarium sulfide in barium thiosamarate and zirconium-containing phases ZrS2 and BaZrS3. According to the mapping data, the CaY2S4–ZrS2 system with ceramic sample conditioning, containing more than 5 mol % ZrS2, contains two phases: CaY2S4 and Y2ZrS5. The ceramic conditioning of composite materials is shown to increase the electrical conductivity by 2–2.5 orders of magnitude as compared to the basic ionic salts; whereas the sol–gel conditioning, only by 1–1.5 orders of magnitude. The study of the contribution of ionic conductivity made it possible to characterize the BaSm2S4–ZrS2 material containing up to 5 mol % of dopant as ionic conductor; containing 10–30 mol % of dopant, a mixed ionic–electronic conductor. In the CaY2S4–ZrS2 composite material, the ionic contribution prevails in its conductivity. The effect of the dopant on the nature of ionic conductivity is investigated. In the CaY2S4–ZrS2 and BaSm2S4–ZrS2 heterogeneous mixtures, the predominant sulfide-ionic transfer of basic barium thiosamarate and calcium thioitrate is retained; however, the fraction of cationic transfer somewhat increased.












Similar content being viewed by others
REFERENCES
Ivanov-Shits, A.K. and Murin, I.V., Ionics of Solids (in Russian), St. Petersburg: St. Petersburg Gos. Univ., 2010. vol. 2.
Ananchenko, B.A., Synthesis and study of electrolytic properties of phases based on calcium thioytterbiate, Cand. Sci. (Chem.) Dissertation, St. Petersburg: St. Petersburg Gos. Univ., 2017, p. 176.
Kalinina, L.A., Shirokova, G.I., Murin, I.V., Ushakova, Yu.N. et al., Sulfide-conducting solid electrolytes, J. Appl. Chem. (in Russian), 2000, vol. 73, no. 8, p. 1324.
Kalinina, L.A., Shirokova, G.I., Lyalina, M.Yu., Chernov, S.V., and Murin, I.V., Electrochemical study of sulphide-conducting solid electrolytes, Sb. “Electrodics of solid-state systems” (in Russian), 1994, p. 18.
Johnson, V.S., Synthesis and characterisation of ceramic potential sulphide conductors, Doctoral (Chem.) Dissertation, Loughborough: Loughborough Univ., UK, 2005.
White, R.J., Synthesis and characterisation of complex sulfide materials with potential use as high temperature inorganic sulfide-ion conductors. Doctoral (Chem.) Dissertation, Loughborough: Loughborough Univ., UK, 2006. https://dspace.lboro.ac.uk/2134/7824
Ananchenko, B.A., Myakishev, A.O., Kalinina, L.A., Kosheleva, E.V., and Murin, I.V., Effect of composition on character of defect formation and ion transport in (1 – x)\([{\text{C}}{{{\text{a}}}_{{1{\text{ }}-y}}}{\text{Yb}}_{y}^{{2 + }}]{\text{Yb}}_{2}^{{3 + }}{{{\text{S}}}_{{4 - d}}}{\text{Y}}{{{\text{b}}}_{2}}{{{\text{S}}}_{3}}\) phases, Russ. J. Electrochem., 2017, vol. 53, p. 799–807.
Uvarov, N.F., Composite Solid Electrolytes (in Russian), Novosibirsk: Russ. Akad. Nauk, Sib. Branch, 2008.
Brune, A., and Wagner, J.B., Electrical conductivity of PbCl2 with a dispersed second phase, Solid State Ionics, 1987, vol. 25, p. 165.
Maier, J., Space charge regions in solid two-phase systems and their conduction contribution. I. Conductance enhancement in the systems ionic conductor—“inert” phase and application on AgCl–Al2O3 and AgBr–Al2O3, J. Phys. Chem. Solids., 1985, vol. 46, p. 309.
Ponomareva, V.G., Tarnopol’skii, V.A., Yaroslavtsev, A.B., and Burgina, E.B., Proton conductivity in H3OFe(SO4)2–SiO2 composites, J. Inorg. Chem. (in Russian), 2003, vol. 48, p. 1061.
Konisheva, E., Neiman, A., and Gorbunova, E., Transport processes and surface transformation at the CaWO4:WO3 interface, Solid State Ionics, 2003, vol. 157, p. 45.
Neiman, A.Ya., Pestereva, N.N, Zhou, Yu.Y., and Nechaev, D.O., The nature and mechanism of ion transport in tungstates Me2+{WO4} (Ca, Sr, Ba) and Me{WO4}3+ (Al, Sc, In) according to the method of Tubandt, Russ. J. Electrochem., 2013, vol. 49, p. 999.
Kosheleva, E.V., Pentin, M.A., Kalinina, L.A., Mikhailichenko, T.V., Lapteva T.A., and Ushakova, Yu.N., Heterogeneous doping of sulfide-conducting phases based on calcium and barium thiolanthanates, Russ. J. Electrochem., 2017, vol. 53, p. 790–798.
Pentin, M.A., Ananchenko, B.A., Kalinina, L.A., Kosheleva, E.V., Ushakova, Yu.N., and Murin, I.V., Sulfide-Conducting ionic conductors with the CaFe2O4 and Yb3S4 structure doped with zirconium disulfide, Russ. J. Electrochem. 2019, vol. 55, p. 785.
Mikhailichenko, T.V., Synthesis of solid electrolytes based on barium thiosamarate and the study of their electrolytic properties, Cand. Sci. (Chem.) Dissertation, St. Petersburg: St. Petersburg Gos. Univ., 2013, p. 171.
Chebotin, V.N. and Perfilev, M.V., Electrochemistry of Solid Electrolytes (in Russian), Moscow: Khimiya, 1978, p. 310.
Kalinina, L.A., The study of the ternary system BaS–ZrS2 with the alleged sulfide conductivity, Cand. Sci. (Chem.) Dissertation, Moscow: Moscow Gos. Univ., 1976, p. 152.
Funding
The study was carried out in the framework of the State Assignment “Independent exploratory development” (no. 1.4539 201718.9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Based on the materials of the report at the 15th International Meeting “Fundamental Problems of Solid State Ionics”, Chernogolovka, 30.11.–07.12.2020.
Rights and permissions
About this article
Cite this article
Pentin, M.A., Kalinina, L.A., Kosheleva, E.V. et al. Research of Composite Materials BaSm2S4–ZrS2, CaY2S4–ZrS2. Russ J Electrochem 57, 840–851 (2021). https://doi.org/10.1134/S1023193521070107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521070107