Abstract
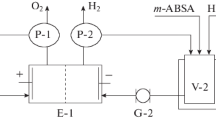
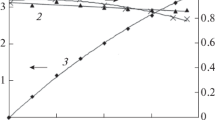
Effect of the naphthalene 2-nitro-4,8-disulfonic acid preparative reduction conditions on the naphthalene 2-amino-4,8-disulfonic acid yield and current efficiency is studied. In particular, the effects of the electrolyte composition, cathode material, current density, temperature, the parent naphthalene 2-nitro-4,8-disulfonic acid concentration are found out. The optimal conditions for the naphthalene 2-amino-4,8-disulfonic acid preparative electrosynthesis in the ammonia-buffer solutions (pH 7.0–8.4) are determined. The naphthalene 2-amino-4,8-disulfonic acid yield and current efficiency of 97.5–98.5% and 65.0–72.2%, respectively, are obtained on scaled-up laboratory unit and pilot plant with a filter-press-type electrolyzer. Based on the experimental data obtained in the scaled-up laboratory unit and pilot plant, the technology of amino-C-acid electrosynthesis is developed. This technology is presented in the experiment-and-industry regulations for the producing of the amino-C-acid and in the requirements specification for designing of a pilot electrolyzer for the current load of 3 kA.










Similar content being viewed by others
REFERENCES
Vorozhtsov, N.N., The Fundamentals of the Intermediate Products and Dyes Syntheses (in Russian), Moscow: Goskhimizdat, 1955.
The Chemistry of Synthetic Dyes, Venkataraman, K. (Ed.), Academic, 1974.
Efros, L.S. and Gorelik, M.V., Chemistry and Technology of Intermediate Products (in Russian), Leningrad: Khimiya, 1980. 544 p.]
Vigasin, A.A., Amitrova, I.M., Ganiushkina, L.S. et al., USSR Inventor’s Certificate no. 154261, 1963.
Bamfield, P., Quan, P.M., and Smith, T.J., US Patent 4093646, 1978.
Hildreth, J.D., US Patent 4299779, 1981.
Horyna, J. and Jehlicka, V., Polarographie der Mononitroderivate der 1,5- und 1,6-Naphthalindisulfonsauren, Coll., 1959, vol. 24, p. 3353.
Fierz, H.E. and Weissenbuch, P., Ueber die Reduktion von Nitronaphtalinssulfosauren, Helv. Chim. Acta, 1920, vol. 3, p. 305.
Konarev, A.A., Electrochemical synthesis of Cleves-acids, Russ. J. Electrochem., 1998, vol. 34, p. 1160.
Konarev, A.A., Katunin, V.Kh., and Sukhinina, N.G., Method for preparation of naphthalene 1-amino-3,6,8-trisulfonic acid, USSR Inventor’s Certificate no. 1369232, 1987.
Konarev, A.A., Avrutskaya, I.A., and Katunin, V.Kh., Polarographic behavior of a naphthalene 1-nitro-3,6,8-trisulfonic acid, Soviet. Electrochem., 1981, vol. 17, p. 901.
Konarev, A.A. and Avrutskaya, I.A., Features of electrochemical reduction of 1-nitro-3,6,8-trisulfonic acid of naphthalene, Soviet. Electrochem., 1988, vol. 24, p. 1548.
Konarev, A.A., RF Patent 2159424, 2000.
Tomilov, A.P., Mayranovsky, S.G., Fioshin, M.Ya., and Smirnov, V.A., Electrochemistry of Organic Compounds (in Russian), Leningrad: Khimiya, 1968.
Konarev, A.A., Selection of anode material for electrosynthesis of naphthalene aminosulfonic acids, Russ. J. Appl. Chem., 1997, vol. 70, p. 261.
Konarev, A.A., Studies of Intermediates Formed under the Conditions of Preparative Electroreduction of Naphthalene 1-Nitro-3,6,8-Trisulfo Acid, Russ. J. Electrochem., 2012, vol. 48, p. 922.
Organic Electrochemistry. An Introduction and a Guide. Baizer, M. and Lund, H., Eds., New York: Marcel Dekker, 1973.
Mizuch, K.G., Collateral oxidation processes in reduction of aromatic nitrocompounds, The USSR Acad. Sci. J., 1937, vol. 15, no.1, p. 37.
Lukashevich, V.O., Organic Semi-products and Dyes (in Russian), Moscow: Goskhimizdat, 1959.
Antropov, L.I., Theoretical Electrochemistry, Univ. Pacific, 2001.
Akhmetov, N.S., General and Inorganic Chemistry (in Russian), Moscow: Vysshaya Shkola, 1981.
Bakir D., Extended Abstract of Cand. Sci. Dissertation, Moscow Gos. Univ., Moscow, 1989.
Smith, J.W. and Waller, J.G., Polarographic Behavior of Aromatic Nitro Compounds Nitrosobenzene and N‑Phenylhydroxylamine, Trans. Farad. Soc., 1950, vol. 46, p. 290.
Stradins, J., Polarography of Organic Nitrocompounds, Riga: Latvian Akad. Nauk, 1961.
Mayranovsky, S.G., Stradins, J., and Bezugliy, V.D., Polarography in Organic Chemistry (in Russian), Leningad: Khimiya, 1975.
Konarev, A.A., RF Patent 2009125, 1994.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Rights and permissions
About this article
Cite this article
Konarev, A.A. Electrochemical Synthesis of Amino-C-Acid. Russ J Electrochem 57, 289–305 (2021). https://doi.org/10.1134/S1023193521030058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521030058