Abstract
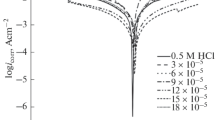
The inhibition characteristics of Trizma for the inhibition of the corrosion of aluminium in 1.0 M HCl solution were studied using weight loss method, potentiodynamic and electrochemical impedance spectroscopy (EIS) techniques. The results indicated that, Trizma act as an efficient mixed type inhibitor retarding both the general and pitting corrosion of aluminium (1 × 10–2 M Trizma gave more than 95% inhibition). The surface techniques SEM and EDX were used to examine and analyze the surface of aluminium after its immersion in 1.0 M HCl in absence and presence of different concentrations of Trizma for 6 h. The results indicated that, the inhibition of corrosion of aluminium by Trizma is controlled by competitive adsorption. The experimental data of the adsorption of Trizma at aluminium surface were fitted to Langmuir isotherm and the Kinetic-thermodynamic model. The results indicated that Langmuir isotherm is not applied, however, the Kinetic-thermodynamic model fits the results and gave a value of \(\Delta G_{{{\text{ads}}}}^{^\circ }\) = –34.9 kJ/mol and adsorption of Trizma on the aluminium surface is (chemical/physical).










Similar content being viewed by others
REFERENCES
Hart, R.K., The oxidation of aluminium in dry and humid oxygen atmospheres, Proc. R. Soc. London. Ser. A: Math. Phys. Sci., 1956, vol. 236, no. 1204, p. 68.
Evan, V.R., The Corrosion and Oxidation of Metals: Specific Principles and Practical Applications, London: Arnold, 1971, p. 319.
Godard, H.P., Jepson, W.P., Bothwell, M.R., and Kane, R.L., The Corrosion of Light Metals, New York: John Wiley & Sons, 1967, p. 1.
Samuels, B.W., Sotoudeh, K., and Foley, R.T., Inhibition and acceleration of aluminum corrosion, Corrosion, 1981, vol. 37, p. 92.
Altenpohl, D., An Introduction into the Metallurgy of Aluminum Fabrication, Dusseldorf: Aluminium-Verlag, 1982, p. 181.
Leckie, H.P. and Uhlig, H.H., Environmental factors affecting the critical potential for pitting in 18–8 stainless steel, J. Electrochem. Soc., 1966, vol. 113, p. 1262.
Kolotyrin, J.M., Pitting corrosion of metals, Corrosion, 1963, vol. 19, p. 261.
Garrigues, L., Pebere, N., and Dabosi, F., An investigation of the corrosion inhibition of pure aluminum in neutral and acidic chloride solutions, Electrochim. Acta, 1996, vol. 41, p. 1209.
Lorking, K.F. and Mayne, J.E.O., The corrosion of aluminium in solutions of sodium fluoride and sodium chloride, Br. Corros. J., 1966, vol. 1, p. 181.
Lorking, K.F. and Mayne, J.E.O., The corrosion of aluminium, J. Appl. Chem., 1961, vol. 11, p. 170.
Edeleanu, C. and Evans, U.R., The causes of the localized character of corrosion on aluminium, Trans. Faraday Soc., 1951, vol. 47, p. 1121.
Nguyen, T.H. and Foley, R.T., The chemical nature of aluminum corrosion II. The initial dissolution step, J. Electrochem. Soc., 1982, vol. 129, p. 27.
El-Awady, A.A., Abd-El-Nabey, B.A., and Aziz, S.G., Thermodynamic and kinetic factors in chloride ion pitting and nitrogen donor ligand inhibition of aluminium metal corrosion in aggressive acid media, J. Chem. Soc., Faraday Trans., 1993, vol. 89, p. 795.
Abdel-Gaber, A.M., Abd-El-Nabey, B.A., Sidahmed, I.M., El-Zayady, A.M., and Saadawy, M., Kinetics and thermodynamics of aluminium dissolution in 1.0 M sulphuric acid containing chloride ions, Mater. Chem. Phys., 2006, vol. 98, p. 291.
Emregül, K.C. and Aksüt, A.A., The effect of sodium molybdate on the pitting corrosion of aluminum, Corros. Sci., 2003, vol. 45, p. 2415.
Silva, J.W.J., Codaro, E.N., Nakazato, R.Z., and Hein, L.R.O., Influence of chromate, molybdate and tungstate on pit formation in chloride medium, Appl. Sur. Sci., 2005, vol. 252, p. 1117.
Abdel Rehim, S.S., Hassan, H.H., and Amin, M.A., Corrosion and corrosion inhibition of Al and some alloys in sulphate solutions containing halide ions investigated by an impedance technique, Appl. Surf. Sci., 2002, vol. 187, p. 279.
Abd El Aal, E.E., Abd El Wanees, S., Farouk, A., and Abd El Haleem, S.M., Factors affecting the corrosion behaviour of aluminium in acid solutions. II. Inorganic additives as corrosion inhibitors for Al in HCl solutions, Corros. Sci., 2013, vol. 68, p. 14.
Yurt, A. and Aykın, Ö., Diphenolic schiff bases as corrosion inhibitors for aluminium in 0.1 M HCl: potentiodynamic polarisation and EQCM investigations, Corros. Sci., 2011, vol. 53, p. 3725.
Şafak, S., Duran, B., Yurt, A., and Türkoğlu, G., Schiff bases as corrosion inhibitor for aluminium in HCl solution, Corros. Sci., 2012, vol. 54, p. 251.
Zhang, Q., Gao, Z., Xu, F., and Zou, X., Adsorption and corrosion inhibitive properties of gemini surfactants in the series of hexanediyl-1,6-bis-(diethyl alkyl ammonium bromide) on aluminium in hydrochloric acid solution, Colloids Surf. A. Physicochem. Eng. Asp., 2011, vol. 380, p. 191.
Abd El Haleem, S.M., Abd El Wanees, S., Abd El Aal, E.E., and Farouk, A., Factors affecting the corrosion behaviour of aluminium in acid solutions. I. Nitrogen and/or sulphur-containing organic compounds as corrosion inhibitors for Al in HCl solutions, Corros. Sci., 2013, vol. 68, p. 1.
Szklarska-Smialowska, Z., Insight into the pitting corrosion behavior of aluminum alloys, Corros. Sci., 1992, vol. 33, p. 1193.
Tadros, A.B. and Abd-el-Nabey, B.A., Inhibition of the acid corrosion of steel by 4-amino-3-hydrazino-5-thio-1,2,4-triazoles, J. Electroanal. Chem. Interfacial Electrochem., 1988, vol. 246, p. 433.
Donnelly, B., Downie, T.C., Grzeskowiak, R., Hamburg, H.R., and Short, D., The effect of electronic delocalization in organic groups R in substituted thiocarbamoyl RCSNH2 and related compounds on inhibition efficiency, Corros. Sci., 1978, vol. 18, p. 109.
Zhang, B., Wang, Y., and Gao, M., Tris (hydroxymethyl) aminomethane-functionalized agarose particles: parameters affecting the binding of bovine serum albumin, J. Sep. Sci., 2012, vol. 35, p. 1406.
Dotson, R.L., Characterization and studies of some four, five and six coordinate transition and representative metal complexes of tris-(hydroxymethyl)-aminomethane, J. Inorg. Nucl. Chem., 1972, vol. 34, p. 3131.
Bai, K.S. and Martell, A.E., The interaction of 2-amino-2-(hydroxymethyl)-1,3-propanediol with copper(II) and nickel (II) ions, J. Inorg. Nucl. Chem., 1969, vol. 31, p. 1697.
Lee, S.M., Sim, K.S., and Lo, K.M., Synthesis, characterization and biological studies of diorganotin(IV) complexes with tris [(hydroxymethyl) aminomethane] Schiff bases, Inorg. Chim. Acta, 2015, vol. 429, p. 195.
Zanoli, L.M., D’Agata, R., and Spoto, G., Functionalized gold nanoparticles for ultrasensitive DNA detection, Anal. Bioanal. Chem., 2012, vol. 402, p. 1759.
Castañeda, M.E., Alegret, S., and Merkoci, A., Electrochemical sensing of DNA using gold nanoparticles, Electroanalysis, 2007, vol. 19, p. 743.
Qian, L.H., Wang, K., and Fang, H.T., Au nanoparticles enhance CO oxidation onto SnO2 nanobelt, Mater. Chem. Phys., 2007, vol. 103, p. 132.
Taton, T.A., Mirkin, C.A., and Letsinger, R.L., Scanometric DNA array detection with nanoparticle probes, Science, 2000, vol. 289, p. 1757.
Staderini, M., González-Fernández, E., and Murray, A.F., A tripod anchor offers improved robustness of peptide-based electrochemical biosensors, Sens. Actuators B. Chem., 2018, vol. 274, p. 662.
Nour, A., Hassan, N., and Refaat, H.M., Effect of reducing agent strength on the growth and thermoelectric performance of nanocrystalline bismuth telluride, Mater. Res. Express, 2018, vol. 5, no. 3.
Rashad, M.M., El-Dissouky, A., and Soliman, H.M., Structure evaluation of bismuth telluride (Bi2Te3) nanoparticles with enhanced Seebeck coefficient and low thermal conductivity, Mater. Res. Innov., 2018, vol. 22, p. 315.
Yadav, P.N.S., Singh, A.K., and Wadhwani, R., Role of hydroxyl group in the inhibitive action of benzoic acid toward corrosion of aluminum in nitric acid, Corrosion, 1999, vol. 55, p. 937.
Fetouh, H.A., Abd-El-Nabey, B.A., Goher, Y.M., and Karam, M.S., An electrochemical investigation in the anticorrosive properties of silver nanoparticles for the acidic corrosion of aluminium, J. Electrochem., 2017, vol. 23, p. 4.
Abdel-Gaber, A.M., Abd-El-Nabey, B.A., and Saadawy, M., The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract, Corros. Sci., 2009, vol. 51, p. 1038.
Li, X., Deng, S., and Fu, H., Inhibition by tetradecylpyridinium bromide of the corrosion of aluminium in hydrochloric acid solution, Corros. Sci., 2011, vol. 53, p. 1529.
Li, X., Deng, S., and Xie, X., Experimental and theoretical study on corrosion inhibition of o-phenanthroline for aluminum in HCl solution, J. Taiwan. Inst. Chem. Eng., 2014, vol. 45, p. 1865.
Deng, S. and Li, X., Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminium in HCl solution, Corros. Sci., 2012, vol. 64, p. 253.
Bessone, J., Mayer, C., Jüttner, K., and Lorenz, W.J., AC-impedance measurements on aluminium barrier type oxide films, Electrochim. Acta, 1983, vol. 28, p. 171.
Brett, C.M., On the electrochemical behaviour of aluminium in acidic chloride solution, Corros. Sci., 1992, vol. 33, p. 203.
Abd El Rehim, S.S., Hassan, H.H., and Amin, M.A., Corrosion inhibition of aluminum by 1,1-(lauryl amido) propyl ammonium chloride in HCl solution, Mater. Chem. Phys., 2001, vol. 70, p. 64.
Noor, E.A., Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid, Mater. Chem. Phys., 2009, vol. 114, p. 533.
Amin, M.A., Mohsen, Q., and Hazzazi, O.A., Synergistic effect of I– ions on the corrosion inhibition of Al in 1.0 M phosphoric acid solutions by purine, Mater. Chem. Phys., 2009, vol. 114, p. 908.
Lenderink, H.J.W., Linden, M.V.D., and De Wit, J.H.W., Corrosion of aluminium in acidic and neutral solutions, Electrochim. Acta, 1993, vol. 38, p. 1989.
Våland, T. and Heusler, K.E., Reactions at the oxide-electrolyte interface of anodic oxide films on aluminum, J. Electroanal. Chem. Interfacial Electrochem., 1983, vol. 149, p. 71.
Abdel-Gaber, A.M., Abd-El-Nabey, B.A., and Sidahmed, I.M., Inhibitive action of some plant extracts on the corrosion of steel in acidic media, Corros. Sci., 2006, vol. 48, p. 2765.
El-Awady, A.A., Abd-El-Nabey, B.A., and Aziz, S.G., Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines, J. Electrochem. Soc., 1992, vol. 139, p. 2149.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Abd-El-Nabey, B.A., El-Housseiny, S., Eldissouky, A. et al. Trizma as an Efficient Inhibitor for the Corrosion of Aluminium in Acid Solutions Containing Chloride Ions. Russ J Electrochem 57, 765–773 (2021). https://doi.org/10.1134/S1023193520120034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520120034