Abstract
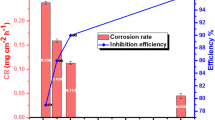
The activity of organoselenides as corrosion inhibitors for mild steel (MS) in 1.0 M hydrochloric acid was evaluated via electrochemical, surface examination and computational chemical methods. The results revealed that the corrosion inhibition percentage (% IE) of organoselenides reached more than 80% with little concentration. Tafel curves showed that organoselenides act as mixed-type inhibitors. EIS measurements indicated that organoselenides mitigate MS corrosion by adsorption on the surface. SEM and AFM analysis depicted the significant improvement in the MS surface in the presence of organoselenides, protecting the surface owing to the formation of an inhibitive film over the metallic surface. Also, DFT computations were conducted on the active form of the investigated compounds. Results from the practical experiments and theoretical calculations suggested that organoselenides are promising inhibitor candidates for corrosion of MS in 1.0 M hydrochloric acid.












Similar content being viewed by others
Change history
02 May 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S1023193523040122
REFERENCES
Prabhu, R.A., Venkatesha, T.V., Shanbhag, A.V., Kulkarni, G.M., and Kalkhambkar, R.G., Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution, Corros. Sci., 2008, vol. 50, p. 3267.
El-Katori, E.E. and Al Angari, Y.M., Electrochemical and theoretical evaluation on the corrosion inhibition of carbon steel by organic selenides in acidic medium, Int. J. Electrochem. Sci., 2018, vol. 13, p. 4319.
Mazher Ahmed Yar, Ying Wang, Xiaorong Zhou, and Constantinos Soutis, Corrosion behaviour of an industrial shot-peened and coated automotive spring steel AISI 9254, Corros. Eng., Sci. Technol., 2018, vol. 53, no. 8, p. 564.
Rybalk, K.V., Beketaev, L.A., and Davydov, A.D., Corrosion behavior of aluminum in 1 M HCl solution, Russ. J. Electrochem., 2016, vol. 52, no. 5, p. 463.
Fakhrul Rozi, Hamed Mohebbi, Mokhtar Che Ismail, Saeid Kakooei, Mahmoud Ahmadi, Amir Aghasadeghi, and Alireza Aghasadeghi, Laboratory investigation on the condensation and corrosion rates of top of line corrosion in carbon steel: a case study from pipeline transporting wet gas in elevated temperature, Corros. Eng., Sci. Technol., 2018, vol. 53, no. 6, p. 444.
Singh, D.D.N., Singh, T.B., and Gaur, B., The role of metal cations in improving the inhibitive performance of hexamine on the corrosion of steel in hydrochloric acid solution, Corros. Sci., 1995, vol. 37, p. 1005.
Ashassi-Sorkhabi, H, Seifzadeh, D, and Hosseini, M.G., EN, EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1 M HCl solution, Corros. Sci., 2008, vol. 50, p. 3363.
Ali, S.A., Saeed, M.T., and Rahman, S.U., The isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic medium, Corros. Sci., 2003, vol. 45, p. 253.
Satapathy, A.K, Gunasekaran, G., Sahoo, S.C., Amit, K., and Rodrigues, P.V., Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution, Corros. Sci., 2009, vol. 51, p. 2848.
Yildirim, A. and Çetin, M., Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors, Corros. Sci., 2008, vol. 50, p. 155.
da Trindade, L.G. and Gonçalves, R.S., Evidence of caffeine adsorption on a low-carbon steel surface in ethanol, Corros. Sci., 2009, vol. 51, p. 1578.
Jordan, L.A., Tschopp, M.A., Mlsna, T.E., Wipf, D., and Horstemeyer, M.F., Modeling and experimental calibration of the corrosion of RHA steel in immersion and salt-fog environments, Corros. Eng., Sci. Technol., 2019, vol. 54, no. 2, p. 114.
Malinowski, S., Jaroszyńska-Wolińska, J., and Herbert, T., Theoretical predictions of anti-corrosive properties of THAM and its derivatives, J. Mol. Model., 2018, vol. 24, p. 1.
Singh, A., Ansari, K.R., Kumar, A., Liu, W., Songsong, C., and Lin, Y., Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment, J. Alloys Compd., 2017, vol. 712, p. 121.
Singh, A., Ansari, K.R., Quraishi, M.A., Lgaz, H. and Lin, Y., Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: theoretical and experimental approaches, J. Alloys Compd., 2018, vol. 762, p. 347.
Al Hamzi, A.H., Zarrok, H., Zarrouk, A., Salghi, R., Hammouti, B., Al-Deyab, S.S., Bouachrine, M., Amine, A., and Guenoun, F., The role of acridin-9 (10H)-one in the inhibition of carbon steel corrosion: thermodynamic, electrochemical and DFT studies, Int. J. Electrochem. Sci., 2013, vol. 8, p. 2586.
Adejoro, I.A., Ojo, F.K., and Obafemi, S.K., Corrosion inhibition potentials of ampicillin for mild steel in hydrochloric acid solution, J. Taibah Univ. Sci., 2015, vol. 9, pp. 196–202.
Bashir, S., Sharma, V., Lgaz, H., Chung, I-M., Singh, A., and Kumar, A., The inhibition action of analgin on the corrosion of mild steel in acidic medium: a combined theoretical and experimental approach, J. Mol. Liq., 2018, vol. 263, p. 454.
Tan, B., Zhang, S., Qiang, Y., Guo, L., Feng, L., Liao, C., Xu, Y., and Chen, S.A., Combined experimental and theoretical study of the inhibition effect of three disulfide-based flavouring agents for copper corrosion in 0.5 M sulfuric acid, J. Colloid Interface Sci., 2018, vol. 526, p. 268.
Verma, C., Obot, I.B., Bahadur, I., Sherif, E.-S.M., and Ebenso, E.E., Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies, Appl. Surf. Sci., 2018, vol. 457, p. 134.
Yadav, M., Behera, D., Kumar, S., and Sinha, R.R., Experimental and quantum chemical studies on the corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl, Ind. Eng. Chem. Res., 2013, vol. 52, p. 6318.
Deyab, M.A., Egyptian licorice extract as a green corrosion inhibitor for copper in hydrochloric acid solution, J. Ind. Eng. Chem., 2015, vol. 22, p. 384.
Hemapriya, V., Prabakaran, M., Parameswari, K., Chitra, S., Kim, S-H., and Chung, I.-M., Dry and wet lab analysis on benzofused heterocyclic compounds as effective corrosion inhibitors for mild steel in acidic medium, J. Ind. Eng. Chem., 2016, vol. 40, p. 106.
He, C., Tian, Z., Zhang, B., Lin, Y., Chen, X., Wang, M., and Li, F., Inhibition effect of environment-friendly inhibitors on the corrosion of carbon steel in recirculating cooling water, Ind. Eng. Chem. Res., 2015, vol. 54, p. 1971.
Obot, I.B., Umoren, S.A., Gasem, Z.M., Suleiman, R. and El Ali, B., Theoretical prediction and electrochemical evaluation of vinylimidazole and allylimidazole as corrosion inhibitors for mild steel in 1 M HCl, J. Ind. Eng. Chem., 2015, vol. 21, p. 1328.
Dohare, P., Ansari, K.R., Quraishi, M.A., and Obot, I.B., Pyranpyrazole derivatives as novel corrosion inhibitors for mild steel useful for industrial pickling process: experimental and quantum chemical study, J. Ind. Eng. Chem., 2017, vol. 52, p. 197.
El-Askalany, A.H., Mostafa, S.I., Shalabi, K., Eid, A.M., and Shaaban, S., Novel tetrazole-based symmetrical diselenides as corrosion inhibitors for N80 carbon steel in 1 M HCl solutions: experimental and theoretical studies, J. Mol. Liq., 2016, vol. 223, p. 497.
Shaaban, S., Negm, A., Sobh, M.A., and Wessjohann, L.A., Organoselenocyanates and symmetrical diselenides redox modulators: design, synthesis and biological evaluation, Eur. J. Med. Chem., 2015, vol. 97, p. 190.
Neese, F., The ORCA program system, Wiley Interdiscip, Rev. Comput. Mol. Sci., 2012, vol. 2, p. 73.
Weigend, F. and Ahlrichs, R., Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, vol. 7, p. 3297.
Fouda, A.S., Ismail, M.A., Temraz, A.M., and Abousalem, A.S., Comprehensive investigations on the action of cationic terthiophene and bithiophene as corrosion inhibitors: experimental and theoretical studies, New J. Chem., 2019, vol. 43, p. 768.
Abousalem, A.S., Ismail, M.A., and Fouda, A.S., A complementary experimental and in silico studies on the action of fluorophenyl-2,2'-bichalcophenes as ecofriendly corrosion inhibitors and biocide agents, J. Mol. Liq., 2019, vol. 276, p. 255.
ElBelghiti, M., Karzazi, Y., Dafali, A., Hammouti, B., Bentiss, F., Obot, I.B., Bahadur, I., and Ebenso, E.E., Experimental, quantum chemical and Monte Carlo simulation studies of 3, 5-disubstituted-4-amino-1, 2, 4-triazoles as corrosion inhibitors on mild steel in acidic medium, J. Mol. Liq., 2016, vol. 218, p. 281.
Sun, H., Ren, P., and Fried, J.R., The COMPASS force field: parameterization and validation for phosphazenes, Comput. Theor. Polym. Sci., 1998, vol. 8, p. 229.
Bunte, S.W. and Sun, H., Molecular modeling of energetic materials: the parameterization and validation of nitrate esters in the COMPASS force field, J. Phys. Chem. B, 2000, vol. 104, p. 2477.
Khaled, K.F., Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors, J. Solid State Electrochem., 2009, vol. 13, p. 1743.
Krishnaveni, K., Sampath, K., Ravichandran, J., and Jayabalakrishnan, C., N-methyl-2-(2-nitrobenzylidene) hydrazine carbothioamide—a new corrosion inhibitor for mild steel 1 mol·L−1 hydrochloric acid, Chin. J. Chem. Eng., 2015, vol. 23, p. 1916.
Rybalka, K.V., Beketaeva, L.A., and Davydov, A.D., Effect of dissolved oxygen on the corrosion rate of stainless steel in a sodium chloride solution, Russ. J. Electrochem., 2018, vol. 54, no. 12, p. 1284.
Prasanna, B.M., Praveen, B.M., Hebbar, N., Pavithra, M.K., Manjunatha, T.S., and Malladi, R.S., Theoretical and experimental approach of inhibition effect by sulfamethoxazole on mild steel corrosion in 1 M HCl, Surf. Interface Anal., 2018, vol. 50, p. 779.
Eldesoky, A.M. and Nozha, S.G., The adsorption and corrosion inhibition of 8-hydroxy-7-quinolinecarboxaldehyde derivatives on C-steel surface in hydrochloric acid, Chin. J. Chem. Eng., 2017, vol. 25, p. 1265.
Xianghong Li, Shuduan Deng, and Xiaoguang Xie, Red tetrazolium as an effective inhibitor for the corrosion of cold rolled steel in 7.0 mol L−1 H2SO4 solution, Chin. J. Chem. Eng., 2018, vol. 26, p. 2641.
Geethanjali, R. and Subhashini, S., Synthesis of water-soluble acryl terpolymers and their anticorrosion properties on mild steel in 1 mol L−1 HCl, Chin. J. Chem. Eng., 2016, vol. 24, p. 543.
Tang, Y., Yang, X., Yang, W., Wan, R., Chen, Y., and Yin, X.A., Preliminary investigation of corrosion inhibition of mild steel in 0.5 M H2SO4 by 2-amino-5-(n-pyridyl)-1,3,4-thiadiazole: polarization, EIS and molecular dynamics simulations, Corros. Sci., 2010, vol. 52, p. 1801.
Fiori-Bimbi, M.V., Alvarez, P.E., Vaca, H., and Gervasi, C.A., Corrosion inhibition of mild steel in HCl solution by pectin, Corros. Sci., 2015, vol. 92, p. 192.
Ayyannan, G., Karthikeyan, K., Vivekananthan, S.S., Gopiraman, M., and Rathinavelu, A., Chemical and electrochemical investigations of high carbon steel corrosion inhibition in 10% HCl medium by quinoline chalcones, Ionics (Kiel), 2013, vol. 19, p. 919.
Fouda, A.S., Ismail, M.A., Abousalem, A.S., and Elewady, G.Y., Experimental and theoretical studies on corrosion inhibition of 4-amidinophenyl-2,2′-bifuran and its analogues in acidic media, RSC Adv., 2017, vol. 7, p. 46414.
Fouda, A.S., Ismail, M.A., Elewady, G.Y., and Abousalem, A.S., Evaluation of 4-amidinophenyl-2,2′-bithiophene and its aza-analogue as novel corrosion inhibitors for CS in acidic media: experimental and theoretical study, J. Mol. Liq., 2017, vol. 240, p. 372.
Sayin, K. and Karakaş, D., Quantum chemical studies on the some inorganic corrosion inhibitors, Corros. Sci., 2013, vol. 77, p. 37.
Arslan, T., Kandemirli, F., Ebenso, E.E., Love, I., and Alemu, H., Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium, Corros. Sci., 2009, vol. 51, p. 35.
Murulana, L.C., Singh, A.K., Shukla, S.K., Kabanda, M.M., and Ebenso, E.E., Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution, Ind. Eng. Chem. Res., 2012, vol. 51, p. 13282.
Saha, S.K., Hens, A., RoyChowdhury, A., Lohar, A.K., Murmu, N.C., and Banerjee, P., Molecular dynamics and density functional theory study on corrosion inhibitory action of three substituted pyrazine derivatives on steel surface, Trans., 2014, vol. 2, p. 489.
Musa, A.Y., Kadhum, A.A.H., Mohamad, A.B., and Takriff, M.S., Molecular dynamics and quantum chemical calculation studies on 4, 4-dimethyl-3-thiosemicarbazide as corrosion inhibitor in 2.5 M H2SO4, Mater. Chem. Phys., 2011, vol. 129, p. 660.
Wang, W., Li, Z., Sun, Q., Du, A., Li, Y., Wang, J., Bi, S., and Li, P., Insights into the nature of the coupling interactions between uracil corrosion inhibitors and copper: a DFT and molecular dynamics study, Corros. Sci., 2012, vol. 61, p. 101.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Emad E. El-Katori, Ashraf S. Abousalem Inhibitive Properties and Computational Approach of Organoselenides on Mild Steel Corrosion in Acidic Environment. Russ J Electrochem 55, 1320–1335 (2019). https://doi.org/10.1134/S1023193519120048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519120048