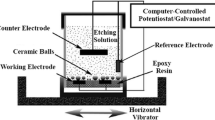
Abstract
The effect of additive DCTA (1,2-diaminocyclohexane-tetraacetic acid) to HCl + H2SO4 solution on electrochemical tunnel etching behavior of aluminum foil was investigated. The results show that DCTA can activate Al foil surface by decreasing the self-corrosion potential and breakdown potential, which may be attributed to that DCTA forms chelate with dissolved Al3+, thus suppressing the oxide film formation. The tunnel density and effective tunnel number as well as uniformity of tunnel length of etching foil can be increased after DCTA addition. Furthermore, the specific capacitance of etching foil can be enhanced with trace addition of DCTA.








Similar content being viewed by others
REFERENCES
Ono, S. and Habazaki, H., Pit growth behaviour of aluminium under galvanostatic control, Corros. Sci., 2011, vol. 53, p. 3521.
Osawa, N. and Fukuoka, K., Pit nucleation behavior of aluminium foil for electrolytic capacitors during early stage of DC etching, Corros. Sci., 2000, vol. 42, p. 585.
Flis, J. and Kowalczyk, L., Effect of sulphate anions on tunnel etching of aluminium, J. Appl. Electrochem., 1995, vol. 25, p. 501.
Hebert, K. and Alkire, R., Growth rates of aluminum etch tunnels, J. Electrochem. Soc., 1988, vol. 135, p. 2447.
Alwitt, R.S., Uchi, H., Beck, T.R., and Alkire, R., Electrochemical tunnel etching of aluminum, J. Electrochem. Soc., 1984, vol. 131, p. 13.
Liang, L., He, Y., Song, H., Yang, X., and Cai, X., Effect of placement of aluminium foil on growth of etch tunnels during DC etching, Corros. Sci., 2014, vol. 79, p. 21.
Hebert, K. and Alkire, R., Growth and passivation of aluminum etch tunnels, J. Electrochem. Soc., 1988, vol. 135, p. 2146.
Kang, J., Shin, Y., and Tak, Y., Growth of etch pits formed during sonoelectrochemical etching of aluminum, Electrochim. Acta, 2005, vol. 51, p. 1012.
Ryu, J.H., Seo, J.H., Jeong, J.H., Kim, S.K., and Dong, N.L., The effect of aluminum ions on the DC etching of aluminum foil, J. Appl. Electrochem., 2004, vol. 34, p. 879.
Hunkeler, F. and Bohni, H., Determination of pit growth rates on aluminum using a metal foil technique, Corrosion-Us, 2012, vol. 37, p. 645.
Hebert, K.R., A mathematical model for the growth of aluminum etch tunnels, J. Electrochem. Soc., 2001, vol. 148, p. B236.
Zhou, Y. and Hebert, K.R., A mathematical model for the initiation of aluminum etch tunnels, J. Electrochem. Soc., 1998, vol. 145, p. 3100.
Novick, S.G., Complexometric titration of zinc, J. Chem. Edu., 1997, vol. 74, p. 1463.
Granholm, K., Harju, L., and Ivaska, A., Desorption of metal ions from kraft pulps. Part 1. Chelation of hardwood and softwood kraft pulp with edta, Bioresources, 2010, vol. 5, p. 206.
Peng, N., He, Y., Song, H., Yang, X., and Cai, X., Effects of electrodeposited Zn nuclei on tunnel etching behavior of aluminum foil, Corros. Sci., 2015, vol. 91, p. 213.
Dunn, C.G., Bolon, R.B., Alwan, A.S., and Stirling, A.W., A scanning electron microscope study of etched aluminum foil for electrolytic capacitors, J. Electrochem. Soc., 1971, vol. 118, p. 381.
Goad, D.G.W. and Uchi, H., Modelling the capacitance of d.c. Etched aluminium electrolytic capacitor foil, J. Appl. Electrochem., 2000, vol. 30, p. 285.
Peng, N., Liang, L., He, Y., Song, H., Yang, X., and Cai, X., Effect of tunnel structure on the specific capacitance of etched aluminum foil, Int. J. Miner. Metall. Mater., 2014, vol. 21, p. 974.
Funding
The work is financially supported by Shenzhen Dongyangguang Industrial Development Co., Ltd.
Author information
Authors and Affiliations
Contributions
The authors of Y. Xiao, Y. Xiang and K. Yu conducted the DC etching experiments. Y. Xiao carried out the theoretical calculations. X. Zhang conducted the electrochemical testing. Y. Xiao, F. He, X. Luo and G. Lyu participated in the writing of the text of the article. All authors participated in the discussion of the results.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yuanlong Xiao, He, F., Zhang, X. et al. Effect of Additive DCTA on Electrochemical Tunnel Etching of Aluminum Foil. Russ J Electrochem 55, 1277–1283 (2019). https://doi.org/10.1134/S1023193519090131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519090131