Abstract
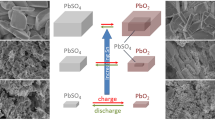
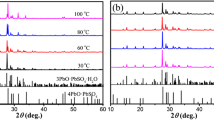
Lead dioxide was extracted from used batteries, and used to synthesize the following intermediate oxides by heating at different temperatures: Pb12O19, Pb12O17, and Pb3O4. Each of the prepared intermediate oxide was subject to sulfuric acid with 1.28 g cm–3. X-ray diffraction (XRD) results showed that the sample prepared from Pb12O19 only had a pattern similar to that of the starting PbO2 with α-PbO2 and β-PbO2 phases. The measurements of H+ proton diffusion coefficient (DH+) of the different samples showed that the sample prepared from Pb12O19 had better electrochemical performances than the starting PbO2. This kinetics reflects the proton insertion mechanism in PbO2, i.e. the sample prepared from Pb12O19 has a large amount of structural water in OH– hydroxyl form. This amount contributes more in the PbO2 reduction mechanism. In addition, the DH+ value of the sample prepared from Pb12O19 is significantly higher than that of starting PbO2, which confirms this hypothesis. X-ray diffraction analysis, thermogravimetric and differential thermogravimetry analysis, and cyclic voltammetry reduction at different scanning rates were used to investigate the samples. This work contributes to environment preservation by recycling of used lead dioxide and reduction of the hazard of its disposal on water.








Similar content being viewed by others
REFERENCES
Fitas, R., Zerroual, L., Chelali, N., and Djellouli, B., Role of hydration water in the reduction process of PbO2 in lead/acid cells, J. Power Sources, 1997, vol. 64, pp. 57–60.
Lin Wei, Xuhui Mao, An Lin, and Fuxing Gan, PbO2–SnO2 composite anode with interconnected structure for the electrochemical incineration of phenol, Russ. J. Electrochem., 2011, vol. 47, pp. 1394–1398.
Brosset, A., Arkiv. Kemi. Mineral. Band. Geol. A, 1945, vol. 20, p. 11.
Pascal, P., Nouveau Traité Chimie Minérale, Paris: Masson, 1960, vol. VIII.
Wyckoff, R.W.G., The Structure of Crystals, New York: Intersci., 1963, vol. 1.
Antonio, P.D. and Santoro, A., Powder neutron diffraction study of chemically prepared β-lead dioxide, Acta Crystallogr. B, 1980, vol. 36, p. 2394.
Santoro, A., Antonio, P.D., and Caulder, S.M., A neutron powder diffraction study of α- and β-PbO2 in the positive electrode material of lead-acid batteries, J. Electrochem. Soc., 1983, vol. 13, p. 1451.
Gavarri, J.R., Garnier, P., and Boher, P., Proton motions in battery lead dioxides, J. Solid State Chem., 1988, vol. 75, p. 251.
Hill, R.J., The crystal structures of lead dioxides from the positive plate of the lead/acid battery, Mater. Res. Bull., 1982, vol. 17, p. 769.
Moseley, P.T., Hutchison, J.L., and Bourke, M.A.M., The defect structure of lead dioxide, J. Electrochem. Soc., 1982, vol. 129, p. 876.
Fitas, R., Zerroual, L., Chelali, N., and Djellouli, B., Thermal degradation of α- and β-PbO2 and its relationship to capacity loss, J. Power Sources, 2000, vol. 85, p. 56.
Fitas, R., Zerroual, L., Chelali, N., and Djellouli, B., Heat treatment of α- and β-battery lead dioxide and its relationship to capacity loss, J. Power Sources, 1996, vol. 58, pp. 225–229.
Fitas, R., Chelali, N., Zerroual, L., and Djellouli, B., Mechanism of the reduction of α- and β-PbO2 electrodes using an all-solid-state system, Solid State Ionics, 2000, vol. 127, p. 49.
Pavlov, D., The lead-acid battery lead dioxide active mass: a gel-crystal system with proton and electron conductivity, J. Electrochem. Soc., 1992, vol. 139, no. 11, p. 3075.
Chahmana, N., Matrakova, M., Zerroual, L., and Pavlov, D., Influence of some metal ions on the structure and properties of doped β-PbO2, J. Power Sources, 2009, vol. 191, pp. 51–57.
Chahmana, N., Zerroual, L., and Matrakova, M., Physicochemical and electrochemical study of lead acid battery positive active mass (PAM) modified by the addition of bismuth, Bulgarian Chem. Commun., 2016, vol. 48, no. 2, pp. 285–289.
Foudia, M., Matracova, M., and Zerroual, L., Effect of a mineral additive on the electrical performances of the positive plate of lead acid battery, J. Power Sources, 2015, vol. 279, pp. 146–150.
Pavlov, D., Hydration and amorphization of active mass PbO2 particles and their influence on the electrical properties of the lead-acid battery positive plate, J. Electrochem. Soc., 1989, vol. 136, no. 11, p. 3189.
Pohl, J.P. and Shendler, W., The electronic conductivity of compact lead dioxide samples with various stoichiometric compositions, J. Power Sources, 1981, vol. 6, p. 245.
Foudia, M., Zerroual, L., and Matracova, M., PbSO4 as a precursor for positive active material electrodes, J. Power Sources, 2012, vol. 207, pp. 51–55.
Noufel, K., Bouzid, A., Chellali, N., and Zerroual, L., Electrochemical performance of γ-MnO2 prepared from the active mass of used batteries, Russ. J. Appl. Chem., 2015, vol. 88, no. 10, pp. 1711–1717.
Dilmi, O. and Benaicha, M., Electrodeposition and characterization of red selenium thin film-effect of the substrate on the nucleation mechanism, Russ. J. Electrochem., 2017, vol. 53, no. 2, pp. 140–147.
Pohl, J.P. and Rickert, H., Elektrochemische Untersuchungen zur Permeation und Löslichkeit von Wasserstoff in Bleidioxid, J. Phys. Chem., 1978, vol. 112, p. 117.
Rüetschi, P. and GIovanoli, R., On the presence of OH− ions, Pb2+ ions and cation vacancies in PbO2, J. Power Sources, 1991, vol. 13, p. 81.
Rüetschi, P., Influence of crystal structure and interparticle contact on the capacity of PbO2 electrodes, J. Electrochem. Soc., 1992, vol. 139, no. 5, pp. 1347–1351.
Caulder, S.M., Murday, J.S., and Simon, A.C., The hydrogen loss concept of battery failure, the PbO2 electrode, J. Electrochem. Soc., 1973, vol. 120, p. 1515.
Hill, R.J. and Jessel, A.M., The electrochemical activity of PbO2 a nuclear magnetic resonance study of hydrogen in battery and chemically prepared material, J. Electrochem. Soc., 1987, vol. 134, p. 1326.
Samoro, A., D’Amonio, P., and Caulder, S.M., A neutron powder diffraction study of α- and β-PbO2 in the positive electrode material of lead-acid batteries, J. Electrochem. Soc., 1983, vol. 130, p. 1451.
Moseley, P.T., Hutchison, J.L., Wright, C.J., Bourke, M.A.M., Hill, R.I., and Rainey, V.S., Inelastic neutron scattering and transmission electron microscope studies of lead dioxide, J. Electrochem. Soc., 1983, vol. 130, p. 829.
Boiler, P., Gamier, P., and Gavarri, J.R., Mise en evidence et localisation des protons dans les bioxydes de plomb PbO2 α et β chimiques et électrochimiques, J. Solid-State Chem., 1984, vol. 52, p. 146.
Gavani, J.R., Gamier, P., Boher, P., Dianoux, A.J., Chedeville, G., and Jacq, B., Proton motions in battery lead dioxides, J. Solid State Chem., 1988, vol. 75, p. 251.
Scherrer, P., Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen, Gttinger Nachrichten, 1918, vol. 2, p. 98.
Matsuda, H. and Ayabe, Y., The theory of the cathode-ray polarography of Randles-Sevcik, Z. Elektrochim. Angew. Phys. Chem., 1955, vol. 59, pp. 494–503.
Münzberg, R. and Pohl, J.P., Proc. 15th Int. Power Sources Symp., Brighton, 1986.
Chelali, N. and Guitton, J., Electrochemical behavior of α- and β-PbO2. Part I: proton diffusion from “all solid-state” protonic electrolyte, Solid State Ionics, 1994, vol. 73, p. 227.
Chelali, N., Zerroual, L., Hammouche, A., and Guitton, J., Electrochemikal behaviour of α- and β-PbO2: Part II: lithium diffusion from non-aqueous electrolyte, Solid State Ionics, 1996, vol. 91, p. 289.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Rahmani, L., Fitas, R., Messai, A. et al. Investigation of Proton Diffusion Coefficient for PbO2 Prepared from Intermediate Oxides. Russ J Electrochem 55, 643–650 (2019). https://doi.org/10.1134/S1023193519070103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519070103