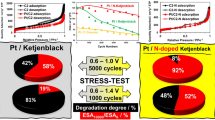
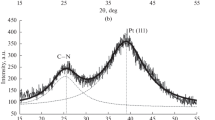
Abstract
Materials containing from 3.1 to 7.7 wt % of cobalt were obtained by electrodeposition of cobalt on Vulcan XC72 carbon powder in suspension. The composition and average diameter of CoO crystallites formed as result of cobalt oxidation in the process of filtering and drying materials, depending on the electrolysis conditions and electrolyte composition, were studied using thermogravimetry and XRD. It is shown that the maximum amount of cobalt can be deposited from electrolytes containing, along with cobalt sulfate, additives of copper and nickel sulfates. Calculations by the Scherrer equation showed that an increase in the CoO content leads to a decrease in the diameter of crystallites, the size of which is in the nano-range. The analysis of X-ray and electrochemical studies indicates the formation, in the course of the borohydride’s synthesis, of combined catalysts containing nanoparticles of the Pt3Co solid solution. The best PtCo/C material demonstrated significant improvement in ORR activity and superior stability compared to commercial Pt/C catalyst of the same platinum loading.
Similar content being viewed by others
References
Guo, Q.Y., Zhang, J.H., Goddard, L., Huang, W.A., and Duan, X.F., Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction, Science, 2016, vol. 354, p. 1414.
Thompsett, D., Catalysts for the Proton Exchange Membrane Fuel Cell. Handbook of Fuel Cells. Fundamentals, Technology and Applications. V 3 Eds. Vielstich W. N.Y.: Wiley, 2003, p. 6.
Alekseenko, A.A., Belenov, S.V., Volochaev, V.A., Novomlinskii, I.N., and Guterman, V.E., CuPt/C-catalysts: synthesis, structure, activity in the oxygen electroreduction reaction, Kondensir. sredy mezhfaznye granitsy (in Russian), 2016, vol. 18, no. 4, p. 460.
Yang, H., Platinum-based electrocatalysts with core-shell nanostructures, Angew. Chem. Int. Ed., 2011, vol. 50, no. 12, p. 2674.
Markovic, N.M., Schmidt, T.J., Stamenlcovic, V., and Ross, P.N., New Electrocatalysts for Fuel Cells, Fuel Cells, 2001, vol. 1, p. 105.
Paulus, U.A., Wokaun, A., Scherer, G.G., Schmidt, T.J., Stamenlcovic, V., Markovic, N.M., and Ross, P.N., Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes, Electrochim. Acta, 2002, vol. 47, p. 3787.
Gasteiger, H.A., Kocha, S.S., Sompalli, B., and Wagner, F.T., Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs, Appl. Catal, B. Environ, 2005, vol. 56, p. 9.
Koh, S., Halm, N., Yu, C., and Strasser, P., Effects of Composition and Annealing Conditions on Catalytic Activities of Dealloyed Pt—Cu Nanoparticle Electro-catalysts for PEMFC, J. Electrochem. Soc., 2008, vol. 155, p. 1281.
Wadayama, T., Yoshida, H., Ogawa, K., Todoroki, N., and Yamada, Y., Outermost Surface Structures and Oxygen Reduction Reaction Activities of Co/Pt(l11) Bimetallic Systems Fabricated Using Molecular Beam Epitaxy, J. Phys. Chem. C, 2011, vol. 115, p. 18589.
Gasteiger, H.A., Kocha, S.S., Sompalli, B., and Wagner, F.T., Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCS, Appl. Catal. B: Environ., 2005, vol. 56, p. 9.
Antolini, E., Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells, J. Mater. Sci., 2003, vol. 38, p. 2995.
Guo, S., Li, D., Zhu, H., Zhang, S., Markovic, N.M., Stamenlcovic, Y.R., and Sun, S., FePt and CoPt Nanowires as Efficient Catalysts for the Oxygen Reduction Reaction, Angew. Chem. Int. Ed., 2013, vol. 52, p. 3465.
Jiang, K., Zhao, D., Guo, S., Zhang, X., Zhu, X., Guo, J., Lu, G., and Huang, X., Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires, Sci. Adv., 2017, vol. 3, p.1601705.
Munoz, M., Ponce, S., Zhang, G.R., Etzold B.J.M., Size-controlled PtNi nanoparticles as highly efficient catalyst for hydrodechlorination reactions, Appl. Catal. B: Environ., 2016, vol. 192, p. 1.
Jalan, V.M. and Taylor, E.J., Importance of Interatomic Spacing in Catalytic Reduction of Oxygen in Phosphoric Acid, J. Electrochem. Soc., 1983, vol. 130, p. 2299.
Toda, T., Igarashi, H., Uchida, H., and Watanabe, M., Enhancement of the Electroreduction of Oxygen on Pt Alloys with Fe, Ni, and Co, J. Electrochem. Soc., 1999, vol. 146, p. 3750.
Beard, B.C. and Ross, P.N., The Structure and Activity of Pt—Co Alloys as Oxygen Reduction Electrocatalysts, J. Electrochem. Soc. 1990. vol. 137, p. 3368.
Paffett, M.T., Berry, J.G., and Gottesfeld, S., Oxygen Reducti on at Pt0.65Cr0.35, Pt0.2Cr0.8 and Roughened Platinum, J. Electrochem. Soc., 1988, vol. 135, p. 1431.
Xiong, L., Kantian, A.M., and Manthiram. A., Pt—M (M = Fe, Co, Ni and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells, Electrochem. Commun., 2002, vol. 4, p. 898.
Stamenlcovic, V., Schmidt, T.J., Ross, P.N., and Markovic, N.M., Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt 3 Ni and Pt 3 Co Alloy Surfaces, J. Phys. Chem., 2002, vol. 106, p. 11970.
Stamenlcovic, V., Schmidt, T.J., Ross, P.N., and Markovic, N.M., Surface segregation effects in electrocatalysis: kinetics of oxygen reduction reaction on polycrystalline Pt3Ni alloy surfaces, J. Electroanal. Chem., 2003, vol. 191, p. 554.
Salgado, J.R.C., Antolini, E., and Gonzalez, E.R., Structure and Activity of Carbon-Supported Pt—Co Electrocatalysts for Oxygen Reduction, J. Phys. Chem. B, 2004, vol. 108, p. 17767.
Min, M., Cho, J., Cho, K., and Kim, H., Particle size and alloying effects of Pt-based alloy catalysts for fuel cell applications, Electrochim. Acta, 2000, vol. 45, p. 4211.
Shukla, A.K., Neergat, M., Bera, P., Jayaram, V., and Hegde, M.S., Catalyst electrode preparation for PEM fuel cells by electrodeposition, J. Electroanal. Chem., 2001, vol. 111, p. 504.
Neergat, M., Shukla, A.K., and Gandhi, K.S., Effects of Heat Treatment on the Catalytic Activity and Methanol Tolerance of Carbon-Supported Platinum Alloys, J. Appl. Electrochem, 2001, vol. 31, p.373.
Antolini, E., Formation of carbon-supported PtM alloys for low temperature fuel cells: a review, Mater. Chem. Phys, 2003, vol. 78, p. 563.
Guterman, A.V., Pakhomova, E.B., Guterman, V.E., Kabirov, Yu.V., and Grigor’ev, V.P., Synthesis of nano-structured PtxNi/C and PtxCo/C catalysts and their activity in the reaction of oxygen electroreduction, Inorg. Mater., 2009, vol. 45, no. 7, p. 767.
Moffat, T.P., Mallett, J.J., and Hwang, Sun-Mi., Oxygen Reduction Kinetics on Electrodeposited Pt, Pt100-xNix, and Pt100-xCox, J. Electrochem. Soc., 2009, vol. 156, p. B238.
Guterman, V.E., Novomlinskii, I.N., Alekseenko, A.A., Belenov, S.V., Tsvetkova, G.G., and Balakshina, E.N., Russian Federation Inventor’s Certificate no. 2616190, 13.04.2017.
Guterman, V.E., Novomlinskii, I.N, Skibina, L.M., and Mauer, D.K., Russian Federation Inventor’s Certificate no. 2656914, 07.06.2018.
Kuriganova, A.B., Leontyeva, D.V., Ivanov, S., Bund, A., and Smirnova, N.V., Electrochemical dispersion technique for preparation of hybrid MOx—C supports and Pt/MOx—C electrocatalysts for low temperature fuel cells, J. Appl. Electrochem., 2016, vol. 46, p. 1245.
Alekseenko, A.A., Guterman, V.E., Volochaev, V.A., and Belenov, S.V., Effect of wet synthesis conditions on the microstructure and active surface area of Pt/C-catalysts, Inorg. Mater., 2015, vol. 51, p. 1258.
Grazulis, S., Daskevic, A., Merkys, A., and Chateigner A., Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration, Nucleic Acids Research, 2012, vol. 40, p. 420.
Guterman, V.E., Lastovina, T.A., Belenov, S.V., Tabachkova, N.Yu., Vlasenko, V.G., Khodos, I.I., and Balakshina, E.N., PtM/C (M = Ni, Cu, or Ag) Electrocatalysts: Effects of Alloying Components on Morphology and Electrochemically Active Surface Areas, J. Solid State Electrochem., 2014, vol. 18, no. 5, p. 1307.
Kirakosyan, S.A., Alekseenko, A.A., Guterman, V.E., Volochaev, V.A., and Tabachkova, N.Yu., Effect of CO atmosphere on morphology and electrochemically active surface area in the synthesis of Pt/C and PtAg/C electrocatalysts, Nanotechnologies in Russia, 2016, vol. 11, no. 5–6, p. 287.
Hasche, F., Oezaslan, M., and Strasser, P., Activity, Stability, and Degradation Mechanisms of Dealloyed PtCu3 and PtCo3 Nanoparticle Fuel Cell Catalysts, Chem.Cat.Chem., 2011, vol. 3, p. 1805.
Hodnik, N., Jozinovi, B., Zorko, M., and Gaberelc, M., Stability of Commercial Pt/C Low Temperature Fuel Cell Catalyst: Electrochemical IL-SEM Study, Acta Chim. Slov., 2014, vol. 61, p. 280.
Alekseenko, A.A., Moguchikh, E.A., Safronenko, O.I., and Guterman, V.E., Durability of de-alloyed PtCu/C-electrocatalysts, Int. J. Hydrogen Energy, 2018, vol. 43(51), p. 22885.
Kozinkin, A.V., Vlasenko, V.G., Kulikova, O.V., Shvachko, O.V., Kozinkin, Yu.A., Vysochina, L.L., Guterman, V.E., and Zubavichus, Ya.V., Electronic and atomic structure of platinum—cobalt nanocatalysts, J. Struct. Chem., 2011, vol. 52, p. S76
Guterman, V.E., Pustovaya, L.E., Guterman, A.V., and Vysochina, L.L., Borohydride synthesis of Ptx—Ni/C-catalysts and study of activity in the oxygen electroreduction reaction, Russ. J. Electrochem., 2007, vol. 43, no. 9, p. 1091.
Moguchikh, E.A., Alekseenko, A.A., Guterman, V.E. et al., Effect of the composition and structure of Pt(Cu)/C electrocatalysts on their stability under different stress test conditions, Russ. J. Electrochem., 2018, vol. 54, no. 11, p. 979.
Alekseenko, A.A., Guterman, V.E., Belenov, S.V., Menshikov, V.S., et al., Pt/C electrocatalysts based on the nanoparticles with the gradient structure, Int. J. Hydrogen Energy, 2018, vol. 43 (7), p. 3676.
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © The Author(s), 2019, published in Elektrokhimiya, 2019, Vol. 55, No. 4, pp. 424–435.
Rights and permissions
About this article
Cite this article
Skibina, L.M., Mauer, D.K., Volochaev, V.A. et al. Nanostructured Cobalt-Containing Carbon Supports for New Platinum Catalysts. Russ J Electrochem 55, 438–448 (2019). https://doi.org/10.1134/S1023193519050136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519050136