Abstract
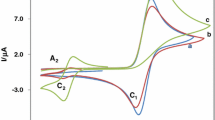
The reaction of electrochemically produced o-benzoquinone from oxidation of catechol as Michael acceptor with L-lysine as nucleophiles has been studied in aqueous solution with various pH values, different electrodes and different concentration of L-lysine using cyclic voltammetry, controlled potential coulometry and differential pulse voltammetry. The participation of reaction of o-benzoquinone with L-lysine at higher concentration of nucleophiles in the second scan of potential was observed. The products generated from the reaction are assumed to be 2-amino-6-((3,4-dihydroxyphenyl)amino)hexanoic acid that undergo electron transfer at more negative potentials than the catechol. The effect of pH of catechol in presence of L-lysine was studied by varying pH from 5 to 11. The reaction was strongly influenced by the pH as well as concentration of L-lysine. The reaction was mostly favorable in 2 mM of catechol and 70 mM of L-lysine at pH 7. The behavior of the reaction mechanism was of electron transfer, chemical reaction and electron transfer (ECE) type.
Similar content being viewed by others
References
Barner, B.A., Catechol, in Encyclopedia of Reagents for Organic Synthesis, Paquette, L., Ed., New York: John Wiley & Sons, 2004.
Khalafi, L. and Rafiee, M., Kinetic study of the oxidation and nitration of catechols in the presence of nitrous acid ionization equilibria, J. Hazard. Mater., 2010, vol. 174, p. 801.
Bisby, R.H., Brooke, R., and Navaratnam, S., Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay, Food Chem., 2008, vol. 108, p. 1002.
Rafiee, M., The electron: the simplest chemical reagent, Synlett, 2007, vol. 3, p. 503.
Nematollahi, D., Rafiee, M., and Fotouhi, L., Mechanistic study of homogeneous reactions coupled with electrochemical oxidation of catechols, J. Iranian Chem. Soc., 2009, vol. 6, p. 448.
PDR for Nonprescription Drugs, Dietary Supplements, and Herbs, 31st ed. Montvale, NJ: PDR Network, LLC, 2010, p. 596.
Lysine, Review of Natural Products. Facts & Comparisons [database online], St. Louis, MO: Wolters Kluwer Health, Feb. 2011.
Motin, M.A., Nazim Uddin, M., Dhar, P.K., Hafiz Mia, M.A., and Hashem, M.A., Voltammetric electro-synthesis of catechol-aspartic acid adduct at different pHs and concentrations, Anal. Bioanal. Electrochem., 2016, vol. 8, p. 505.
Motin, M.A., Alim Uddin, M., Nazim Uddin, M., Dhar, P.K., Hafiz Mia, M.A., and Hashem, M.A., Electrochemical oxidation of catechol in the presence of sulfanilic acid at different pH, Port. Electrochim. Acta, 2017, vol. 35, p. 103.
Hafiz Mia, M.A., Motin, M.A., Huque, E.M., Nazim Uddin, M., Dhar, P.K., and Hashem, M.A., Electro-oxidation of catechol in the presence of L-glutamine at different pH and concentrations, Anal. Bioanal. Electrochem., 2017, vol. 9, p. 597.
Khalafi, L., Rafiee, M., Shahbak, M., and Shirmo-hammadi, H., Kinetic study of the oxidation of cate-chols in the presence of N-methylaniline, J. Chem., 2013, vol. 2013, p. 1.
Shahrokhian, S. and Hamzehloei, A., Electrochemical oxidation of catechol in the presence of 2-thiouracil: application to electro-organic synthesis, Electrochem. Commun., 2003, vol. 5, p. 706.
Nematollahi, D. and Golabi, S.M., Investigation of the electromethoxylation reaction. Part 2: electrochemical study of 3-methylcatechol and 2,3-dihydroxybenzalde-hyde in methanol, Electroanalysis, 2001, vol. 13, p. 1008.
Nematollahi, D. and Goodarzi, H., Electrochemical study of catechol and some of 1,3-diethyl-2-thio-barbituric acid. Application to the electro-organic synthesis of new dispirothiopyrimidine derivatives, J. Electroanal. Chem., 2001, vol. 510, p. 108.
Tabakovic, I., Grujic, Z., and Bejtovic, Z., Electrochemical synthesis of heterocyclic compounds. XII. Anodic oxidation of catechol in the presence of nucleophiles, J. Heterocycl. Chem., 1983, vol. 20, p. 635.
Nematollahi, D. and Forooghi, Z., Electrochemical oxidation of catechols in the presence of 4-hydroxy-6-methyl-2-pyrone, Tetrahedron, 2002, vol. 58, p. 4949.
Golabi, S.M., Nourmohammadi, F., and Saadnia, A., Electrochemical synthesis of organic compounds: 1. Addition of sulfinic acids to electrochemically generated o- and p-benzoquinones, J. Electroanal. Chem., 2002, vol. 529, p. 12.
Janeiro, P., Maria, A., and Brett, O., Catechin electrochemical oxidation mechanisms, Anal. Chim. Acta, 2004, vol. 518, p. 109.
Masek, A., Chrzescijanska, E., and Zaborski, M., Electrochemical properties of catechin in non-aqueous media, Int. J. Electrochem. Sci., 2015, vol. 10, p. 2504.
Khalafi, L., Rafiee, M., and Yadaei, F., Voltammetric study of the oxidation of quercetin and catechin in the presence of cyanide ion, Res. Chem. Intermed., 2011, vol. 37, p. 1047.
Kiani, A., Raoof, J.B., Nematollahi, D., and Ojania, R., Electrochemical study of catechol in the presence of dibuthylamine and diethylamine in aqueous media: Part 1. Electrochemical investigation, Electroanalysis, 2005, vol. 17, p. 1755.
Young, T.E., Griswold, J.R., and Hulbert, M.H., Kinetics of the oxidative cyclization of dopa to dopaquinone, J. Org. Chem., 1974, vol. 39, p. 1980.
Nematollahi, D. and Golabi, S.M., Investigation of the electro-methoxylation reaction: Part 1. Electrochemical study of 4-tert-butylcatechol and 3,4-dihydroxy-benzaldehyde in methanol, J. Electroanal. Chem., 2000, vol. 481, p. 208.
Nematollahi, D., Afkhami, A., Mosaed, F., and Rafiee, M., Investigation of the electro-oxidation and oxidation of catechol in the presence of sulfanilic acid, Res. Chem. Intermedial, 2004, vol. 30, p. 299.
Belenky, P., Bogan, K.L., and Brenner, C., NAD+ metabolism in health and disease, Trends Biochem. Sci., 2007, vol. 32, p. 9.
Mazzini, S., Monderelli, R., Ragg, E., and Scaglioni, L., Interaction between metal ions and NAD(P) coenzymes. 1H, 31P, 13C and 59Co NMR Spectroscopy and conformational analysis, J. Chem. Soc. Perkin Trans., 1995, vol. 2, p. 285.
Rayn, M.D., Yueh, A., and Wen-Yu, C., The electrochemical oxidation of substituted catechols, J. Electrochem. Soc., 1980, vol. 127, p. 1489.
Pasta, M., Mantia, F.L., and Cui, Y., Mechanism of glucose electrochemical oxidation on gold surface, Electrochim. Acta, 2010, vol. 55, p. 5561.
Acknowledgments
Thanks to Ministry of Science and Technology, Government of the People’s Republic of Bangladesh and KUET for providing necessary facilities and financial support to this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2019, Vol. 55, No. 5, pp. 535–545.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Hafiz Mia, M.A., Motin, M.A. & Huque, E.M. Electrochemical Oxidation of Catechol in the Presence of L-Lysine at Different pH. Russ J Electrochem 55, 370–380 (2019). https://doi.org/10.1134/S1023193519050070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519050070