Abstract
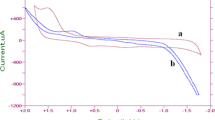
In this study, a grafted polymer (GP) with ZnO nanoparticles (GP/ZnO NPs) was attached on the surface of glassy carbon electrode (GCE), in order to produce a new modified electrode (GP/ZnO NPs-GCE). The gamma irradiation method was used to grafted polystyrene (polymer) with acrylonitrile (monomer), while slow evaporation process was used to prepare the new modified electrode. The cyclic voltammetry (CV) of K4[Fe(CN)6] was used to study the electrochemical properties GP/ZnO NPs-GCE. The peak separation (ΔEpa-c) was 500 mV between the redox peaks of Fe(II)/Fe(III) in an aqueous solution of 1 M KCl and the current ratio of redox current peaks (Ipa/Ipc) was ≈ 1 for the modified electrode. This indicated that the modified electrode has s good reversibility and conductivity, wherefore; it was applied in the voltammetric filed. It was found that the modified electrode GP/ZnO NPs-GCE have a reasonable solubility and stability at various pH medium. Additionally, the sensitivity of the electrochemical analysis by cyclic voltammetric (CV) method is extensively subjected to the pH medium and the scan rate (SR). A couple of redox current peaks of K4[Fe(CN)6] in KCl solution was observed with a reversible process: Fe3+/Fe2+. Finally a good diffusion coefficient of electroactive species (D) for the new modified electrode was found in this study by chronoamperometry method using Cottrell equation.
Similar content being viewed by others
References
Radhi, M.M., Tan, W.T., Ab Rahman, M.Z.B., and Kassim, A.B., Electrochemical characterization of the redox couple of [Fe(CN)6]3–Fe(CN)6]4–mediated by a graft polymer modified glassy carbon electrode, J. Chem. Eng. Jpn., 2010, vol. 43, pp. 927–931.
Labaye, D.E., Jerome, C., Gueskin, V.M., Louette, P., Lazzaroni, R., Martinot, L., and Jerome, R., Full electrochemical synthesis of conducting polymer films chemically graft to conducting surfaces, J. Am. Chem. Soc., 2002, vol. 18, pp. 5222–5230.
Peter, M.L., Rob, G.H., Hempenius, M.A., and Vancso, J., Electrochemistry of surface-graft stimulusresponsive monolayers of poly(ferrocenyldimethylsilane) on gold, Langmuir, 2005, vol. 21, pp. 5115–5123.
Chuy, C., Ding, J., Swanson, E., Holdcroft, S., Horsfall, J., and Lovell, K.V., Conductivity and electrochemical ORR mass transport properties of solid polymer electrolytes containing poly(styrene sulfonic acid) graft chains, J. Electrochem. Soc., 2003, vol. 150, pp. 271–279.
Radhi, M.M., Tan, W.T., and Haider, A.J., Electrochemical characterization of the redox couple of Fe(II)/Fe(III) mediated by a graft polymer electrode in nonaqueous electrolyte, Int. J. Electrochem. Sci., 2012, vol. 7, pp. 1–10.
Xiong, H.-M., Wang, Z.-D., Xie, D.-P., Cheng, L., and Xa, Y.-Y., Stable polymer electrolytes based on polyether-graft ZnO nanoparticles for all-solid-state lithium batteries, J. Mater. Chem., 2006, vol. 16, pp. 1345–1349.
Idris, M.R. and Quayum, E.M., Preparation of ZnO nanoparticles and its electrochemical studies, Nano Vision, 2013, vol. 2, pp. 76–83.
Ramanavicius, A., Oztekin, Y., Balevicius, Z., Kausaite-Mikstimiene, A., Krikstolaityte, V., Baleviciute, I., Ratautaite, V., and Ramanaviciene, A., Conducting and electrochemically generated polymers in sensor design, Procedia Eng., 2012, vol. 47, pp. 825–828.
Starowicz, M. and Stypula, B., Electrochemical synthesis of ZnO nanoparticles, Eur. J. Inorg. Chem., 2008, vol. 6, pp. 869–872.
Xiong, H.-M., Liu, D.-P., Xia, Y.-Y., and Chen, J.-Sh., Polyether-graft ZnO nanoparticles with tunable and stable photoluminescence at room temperature, Chem. Mater., 2005, vol. 12, pp. 3062–3064.
Fang, L., Zhang, B., Li, W., Zhang, J., Huang, K., and Zhang, Q., Fabrication of highly dispersed ZnO nanoparticles embedded in graphene nanosheets for high performance supercapacitors, Electrochim. Acta, 2014, vol. 148, pp. 164–169.
Hong, R.Y., Chen, L.L., Li, J.H., Zheng, Y., and Ding, J., Preparation and application of polystyrenegraft ZnO nanoparticles, Polym. Adv. Technol., 2007, vol. 18, pp. 901–909.
Achilleos, D.S. and Vamvakaki, M., End-graft polymer chains onto inorganic nano-objects, Materials, 2010, vol. 3, pp. 1981–2026.
Li, Sh., Lin, M.M., Toprak, M.S., Kim, D.K., and Muhammed, M., Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications, Nano Rev., 2010, vol. 1, pp. 1–19.
El-Said, W.A. and Choi, J.-W., Electrochemical biosensor consisted of conducting polymer layer on gold nanodots patterned indium tin oxide electrode for rapid and simultaneous determination of purine bases, Electrochim. Acta, 2014, vol. 123, pp. 51–57.
Radhi, M.M., Al-Damlooji, N.K., Radhi Jobayr, M., and Dawood, D.S., Electrochemical sensors of cyclic voltammetry to detect Cd(II) in blood medium, sensors, Transducers, 2013, vol. 155, pp. 150–154.
Tan, W.T., Radhi, M.M., Rahman, M.Z.B., and Kassim, A., Synthesis and characterization of graft polystyrene with acrylonitrile using gamma-irradiation, J. Appl. Sci., 2010, vol. 10, pp. 139–144.
Tan, W.T., Farhan, Y., and Zulkarnain, Z., Electrochemical reduction of potassium ferricyanide mediated by magnesium diboride modified carbon electrode, Sensors Transducers J., 2009, vol. 104, pp. 119–127.
Bard, A.J. and Larry, L.R., Electrochemical Methods: Fundamentals and Applications, 2nd ed., New York: Wiley, 2000.
Zhang, J., Electrochemical methods: Fundamental and applications, Electro Anal. Chem., 1972, vol. 331, pp. 945–957.
Instruction Manual, CV 50W, ver. 2, West Lafayette: Bioanalytical System, 1996.
Radhi, M.M., Amir, Y.K.A., Alwan, S.H., and Tee, T.W., Electrochemical effect of different modified glassy carbon electrodes on the values of diffusion coefficient for some heavy metal ions, J. Phys.: Conf. Ser., 2013, vol. 431, pp. 1–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2018, Vol. 54, No. 1, pp. 33–39.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Radhi, M.M., Alosfur, F.K.M. & Ridha, N.J. Voltammetric Characterization of Grafted Polymer Modified with ZnO Nanoparticles on Glassy Carbon Electrode. Russ J Electrochem 54, 27–32 (2018). https://doi.org/10.1134/S1023193518010068
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193518010068