Abstract
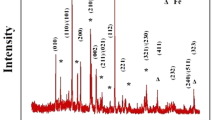
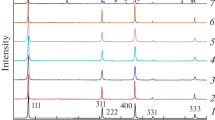
Different approaches to synthesis of Li2FeSiO4-based electrode materials for lithium intercalation, using low-cost and abundant Li-, Si-, and Fe-containing parent substances, are discussed. XRD, SEM, and a laser-diffraction analyzer of particle size were used for structure and morphology characterization of the composite electrode materials. Li2FeSiO4 was shown to be the main lithium-accumulating crystalline phase; minor LiFeO2 and Li2SiO3 admixtures are also present. The material microparticles’ average size was shown to vary from tenths of micrometer to 1 μm. Larger objects sized ca. 2–4 μm are the microparticles’ agglomerates. The material electrochemical properties were studied by dc chronopotentiometry (galvanostatic charging–discharging) and cyclic voltammetry with potential linear sweeping. The initial reversible cycled capacity of the best samples is 170 mA h/g. The anodic and cathodic processes manifest obvious hysteresis caused by the presence of several different lithium ion energy states in the material; the transition between the states is kinetically hindered. The dependences of the specific capacity and its stability under cycling on the current load and the conductive carbon component content in the composite were elucidated.
Similar content being viewed by others
References
Tarascon, J.-M. and Armand, M., Nature, 2001, vol. 414, p. 359.
Padhi, A.K., Nanjundaswamy, K.S., and Goodenough, J.B., J. Electrochem. Soc., 1997, vol. 144, p. 1188.
Lyness, C., Delobel, B., Armstrong, A.R., and Bruce, P.G., Chem. Commun., 2007, vol. 46, p. 4890.
Dominko, R., J. Power Sources, 2008, vol. 184, p. 462.
Nytén, A., Abouimrane, A., Armand, M., Gustafsson, T., and Thomas, J.O., Electrochem. Commun., 2005, vol. 7, p. 156.
Belharouak, I., Abouimrane, A., and Amine, K., J. Phys. Chem., 2009, vol. 113, p. 20733.
Gong, Z.L., Li, Y.X., He, G.N., Li, J., and Yang, Y., Electrochem. Solid-State Lett., 2008, vol. 11, p. A60.
Kokalj, A., Dominko, R., Mali, G., Meden, A., Gaberscek, M., and Jamnik, J., Chem. Mater., 2007, vol. 19, p. 3633.
Rangappa, D., Murukanahally, K.D., Tomai, T., Unemoto, A., and Honma, I., Nano Lett., 2012, vol. 12, p. 1146.
Arroyo-de Dompablo, M.E., Armand, M., Tarascon, J.-M., and Amador, U., Electrochem. Commun., 2006, vol. 8, p. 1292.
Gong, Z.L., Li, Y.X., and Yang, Y., J. Power Sources, 2007, vol. 174, p. 524.
Fan, X.-Y., Li, Y., Wang, J.-J., Gou, L., Zhao, P., Li, D.-L., Huang, L., and Sun, S.-G., J. Alloys Comp., 2010, vol. 493, p. 77.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Ivanishchev, A.V. Churikov, A.S. Akmaev, A.V. Ushakov, I.A. Ivanishcheva, I.M. Gamayunova, M.J. Sneha, A. Dixit, 2017, published in Elektrokhimiya, 2017, Vol. 53, No. 3, pp. 340–351.
Rights and permissions
About this article
Cite this article
Ivanishchev, A.V., Churikov, A.V., Akmaev, A.S. et al. The synthesis, structure, and electrochemical properties of Li2FeSiO4-based lithium-accumulating electrode material. Russ J Electrochem 53, 302–311 (2017). https://doi.org/10.1134/S1023193517030089
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193517030089