Abstract
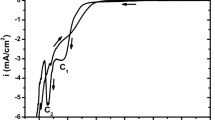
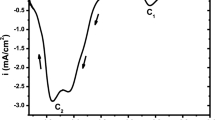
In this work, cyclic voltammetry (CV) and chronoamperometry (CA) were used to study the electrodeposition mechanism of red selenium on platinum and (ITO) substrates from aqueous solution containing (SeO2) and sodium citrate as support electrolyte with pH 4.3 at ambient temperature. The potentiostatic current transients were analyzed according to Scharifker–Hills model. The morphological characterization of the deposit was carried out by Scanning Electron Microscopy (SEM), whereas the optical one was realized by UV-Visible spectroscopy. The results shown that the nucleation mechanism of Se on each substrate is instantaneous with a three-dimensional growth of the hemispherical nuclei. The nucleation density (N 0) is exponentially increased with the applied overpotential. Se thin film has an energy gap of about 2.4 eV.
Similar content being viewed by others
References
Randey, R.K., Sahu, S.N., and Chandra, S., Handbook of Semiconductor Electrodeposition, N.-Y.: Marcel Dekker Inc., 1996.
Johnson, J.A., Saboungi, M.L., Thiyagarajan, P., Scencsits, R., and Meisel, D., J. Phys. Chem. B, 1999, vol. 103, p. 59.
Gates, B., Mayers, B., Cattle, B., and Xia, Y.N., Adv. Funct. Mater., 2002, vol. 12, p. 219.
Tan, S.H. and Kounaves, S.P., Electroanalysis, 1998, vol. 10, p. 364.
Ferri, T. and Sangiorgio, P., Anal. Acta, 1999, vol. 385, p. 377.
Badr, Y. and Mahmoud, M.A., Physica B, 2005, vol. 369, p. 278.
Remigiusz Kowalik and Krzysztof Fitzner, J. Electroanal. Chem., 2009, vol. 633, p. 78.
Zein El Abedin, S., Saad, A.Y., Farag, H.K., Borisenko, N., Liu, Q.X., and Endres, F., Electrochim. Acta, 2007, vol. 52, p. 2746.
Yanqing Lai, Fandyang Liu, Jie Li, Zhian Zhang, and Yexiang Liu, J. Electroanal. Chem., 2010, vol. 639, p. 187.
Kargar Razi, M., Maamoury, R.S., and Banihashemi, S., Int. J. Nano. Dim., 2011, vol. 1, p. 261.
Gurin, V.S., Prokopenko, V.B., Alexeenko, A.A., Wang, Sh., and Prokoshin, P.V., J. Mater. Sci. Eng. C, 2001, vol. 15, p. 93.
Haiqing Jiang, Xi Yao, Jun Che, Minqiang Wang, and Fantao Kong, J. Ceram. Int., 2004, vol. 30, p. 1685.
Wandong Zhang, Yamin Chai, Nana Cao, and Yonglan Wang, J. Mater. Lett., 2014, vol. 134, p. 123.
Yuan-tao Chen, Wei Zhang, Yan-qing Fan, Xiao-qing Xu, and Zhong-xin Zhang, J. Mater. Chem. Phys., 2006, vol. 98, p. 191.
Zhenghua Wang, Xiangying Chen, Jianwei Liu, Xiaogang Yang, and Yitai Qian, J. Inorg. Chem. Commun., 2003, vol. 6, p. 1329.
Martínes-Escobar, D., Ramachandran Manoj, Sánchez-Juárez, A., Naroo Rios, and Jorge Sergio, J. Thin. Solid Films, 2013, vol. 535, p. 390.
Ubale, A.U. and Sakhare, Y.S., J. Vacuum, 2014, vol. 99, p. 124.
Xuchuan Jiang, Brian Mayers, Yuliang Wang, Bryan Cattle, and Younan Xia, J. Chem. Phys. Lett., 2004, vol. 385, p. 472.
Sheng-Yi Zhang, Juan Zhang, Yi Liu, Xiang Ma, and Hong-Yuan Chen, Electrochim. Acta, 2005, vol. 50, p. 4365.
Josef Pola, Zdenek Bastl, Jan Subrt, and Akihiko Ouchi, J. Appl. Surf. Sci., 2001, vol. 172, p. 220.
Mendoza, D., Lbpez, S., Granandos, S., Morales, F., and Escudero, R., J. Synth. Metals, 1997, vol. 89, p. 71.
Abdel Aal, A., Voigts, F., Chakarov, D., and Endres, F., Electrochim. Acta, 2012, vol. 59, p. 228.
Bartosz Maranowski, Marcin Strawski, Wojciech Ososwiecki, and Marek Szklarczyk, J. Electroanal. Chem., 2015, vol. 752, p. 54.
Cabral Murilo, F., Suffredini Hugo, B., Pedrosa Valber, A., Tanimotoa Sonia, T., and Machado Sergio, A.S., J. Appl. Surf. Sci., 2008, p. 5612.
Steichen, M. and Dale, Ph., J. Electrochem. Commun., 2011, vol. 13, p. 865.
Sheng-Yi Zhang, Juan Zhang, Yi Liu, Xiang Ma, and Hong-Yan Chem, Electrochim. Acta, 2005, vol. 50, p. 4365.
Cattarin, S., Furlanetto, F., and Musiani, M.M., J. Electroanal. Chem., 1996, vol. 415, p. 123.
Scharifker, B. and Hills, G., Electrochim. Acta, 1983, vol. 28, p. 879.
Gunawardena, G., Hills, G., Montenegro, T., and Scharifker, B., J. Electroanal. Chem., 1982, vol. 138, p. 225.
Santos Mauro, C. and Machado Sergio, A.S., J. Electroanal. Chem., 2004, vol. 567, p. 203.
Cavallini, M., Aloisi, G., and Guidelli, R., Langmuir, 1999, vol. 15, p. 2993.
Senthikumar, M., Mathiyarasu, J., Joseph James, Phani, K.L.N., and Yegnaraman, V., Mater. Chem. Phys., 2003, vol. 108, p. 403.
Cerisier, M., Attenborough, K., Celis, J.P., and Van Haesendonck, C., Appl. Surf. Sci., 2000, vol. 166, p. 154.
Gomez, E., Pollina, R., and Vallés, E., J. Electroanal. Chem., 1997, vol. 397, p. 111.
Sauthampton Electrochemistry Group, in Instrumental Methods in Electrochemistry, Kemp, T.J., Ed., Ellis UK, Horwood Ltd., Chichester, 1985.
Grujicic, D. and Pesie, B., Electrochim. Acta, 2004, vol. 29, p. 4719.
Floate, S., Hyde, M., and Compton, R.G., J. Electroanal. Chem., 2002, vol. 523, p. 49.
Budevski, E., Staikov, G., and Lorenz, W.J., Electrochemical Phase Formation and Growth, Weinheim: VCH, 1996.
Volmer, M., Kinetics of Phase Formation, Dresde: Steinkopff, 1939.
Hagfeldt, A. and Grätzel, M., Chem. Rev., 1995, vol. 95, p. 49.
Gonzalez-Hernandez, J., Gorley, P.M., Holrley, P.P., Vartsabyuk, O.M., and Vorobiev, Yu.V., Thin Solid Films, 2002, vol. 403–404, p. 471.
Yamaguchi, T., Yamamoto, Y., Tanaka, T., Tanashi, N., and Yoshida, A., Sol. En. Mate. Sol. Cells, 1998, vol. 50, p. 1.
Huang, C.J., Meen, T.H., Lai, M.Y., and Chen, W.R., Sol. En. Mater. Sol. Cells, 2004, vol. 82, p. 553.
Sadigov, M.SW., Ozkan, M., Bacaksiz, E., Altunbas, M., Kopya, A.I., J. Mater. Sci., 1999, vol. 34, p. 4579.
Singh, R.P., Singh, S.L., and Chandra, S., J. Phys. D: Appl. Phys., 1986, vol. 19, p. 1299.
Pejova, B. and Grozdanov, I., Appl. Surf. Sci., 2001, vol. 1777, p. 152.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2017, Vol. 53, No. 2, pp. 157–164.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Dilmi, O., Benaicha, M. Electrodeposition and characterization of red selenium thin film—effect of the substrate on the nucleation mechanism. Russ J Electrochem 53, 140–146 (2017). https://doi.org/10.1134/S1023193517020045
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193517020045