Abstract
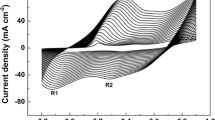
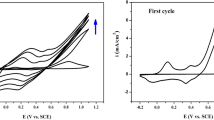
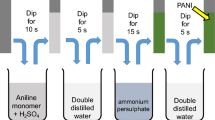
In this work four polyaniline (PANI) film electrode with different thickness were synthesized by electrochemical method on the surface of glassy carbon (GC) electrode. Four polymer films with various thicknesses from 0.5 to 11 μm were synthesized. Electropolymerization occurs in low monomer concentration. Morphology study of electrode shows that surface structure of polymers depends on film thickness. Capacitance of electrode was studied by CV and charge-discharge (CD) methods. Specific capacitance (SC) of electrodes using cyclic voltammetry were calculated 620, 247 F g–1 for thinnest and thickest polymer film, respectively. Stability of electrodes was studied during 1000 voltammogram cycles. Results show that with the increase of thickness the stability of electrodes enhanced and reach to a maximum and then decreased.
Similar content being viewed by others
References
Kötz, R. and Carlen, M., Principles and applications of electrochemical capacitors, Electrochimica Acta, 2000, vol. 45, no. 15, pp. 2483–2498.
Miller, J.R. and Simon, P., Electrochemical capacitors for energy management, Science Magazine, 2008, vol. 321, no. 5889, pp. 651–652.
Yuan, L., Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure, Acs Nano, 2011, vol. 6, no. 1, pp. 656–661.
Frackowiak, E., Supercapacitors based on conducting polymers/nanotubes composites, Journal of Power Sources, 2006, vol. 153, no. 2, pp. 413–418.
Heinze, J.R., Frontana-Uribe, B.A., and Ludwigs, S., Electrochemistry of conducting polymers—persistent models and new concepts, Chemical Reviews, 2010, vol. 110, no. 8, pp. 4724–4771.
Snook, G.A., Kao, P., and Best, A.S., Conductingpolymer- based supercapacitor devices and electrodes, Journal of Power Sources, 2011, vol. 196, no. 1, pp. 1–12.
Wu, Q., Supercapacitors based on flexible graphene/polyaniline nanofiber composite filmsL, ACS Nano, 2010, vol. 4, no. 4, pp. 1963–1970.
Gupta, V. and Miura, N., High performance electrochemical supercapacitor from electrochemically synthesized nanostructured polyaniline, Materials Letters, 2006, vol. 60, no. 12, pp. 1466–1469.
Ryu, K.S., Symmetric redox supercapacitor with conducting polyaniline electrodes, Journal of Power Sources, 2002, vol. 103, no. 2, pp. 305–309.
Sivakkumar, S., Electrochemical performance of polyaniline nanofibres and polyaniline/multi-walled carbon nanotube composite as an electrode material for aqueous redox supercapacitors, Journal of Power Sources, 2007, vol. 171, no. 2, pp. 1062–1068.
Shayeh, J.S., Conductive polymer/reduced graphene oxide/Au nano particles as efficient composite materials in electrochemical supercapacitors, Applied Surface Science, 2015, vol. 353, pp. 594–599.
Shayeh, J.S., Norouzi, P., and Ganjali, M.R., Studying the supercapacitive behavior of a polyaniline/nanostructural manganese dioxide composite using fast Fourier transform continuous cyclic voltammetry, RSC Advances, 2015, vol. 5, no. 26, pp. 20446–20452.
Gobal, F. and Faraji, M., Electrodeposited polyaniline on Pd-loaded TiO2 nanotubes as active material for electrochemical supercapacitor, Journal of Electroanalytical Chemistry, 2013, vol. 691, pp. 51–56.
Hu, C.-C. and Chu, C.-H., Electrochemical impedance characterization of polyaniline-coated graphite electrodes for electrochemical capacitors—effects of film coverage/thickness and anions, Journal of Electroanalytical Chemistry, 2001, vol. 503, no. 1, pp. 105–116.
Shabani, J., Continuous Fast Fourier transform admittance voltammetry as a new approach for study the change morphology of polyaniline for supercapacitors application, RSC Advances, 2015.
Shabani-Shayeh, J., Ehsani, A., and Jafarian, M., Physioelectrochemical investigation of electrocatalytic activity of modified carbon paste electrode in alcohol oxidation as anode in fuel cell, Journal of the Korean Electrochemical Society, 2014, vol. 17, no. 3, pp. 179–186.
Chen, W.-C., Wen, T.-C., and Teng, H., Polyanilinedeposited porous carbon electrode for supercapacitor, Electrochimica Acta, 2003, vol. 48, no. 6, pp. 641–649.
Li, J., The application and research evolvement of the conductive polyaniline in the area of supercapacitor, Materials Review, 2006, vol. 12, p. 006.
MacDiarmid, A., Polyaniline: a new concept in conducting polymers, Synthetic Metals, 1987, vol. 18, no. 1, pp. 285–290.
Göpferich, A., Mechanisms of polymer degradation and erosion, Biomaterials, 1996, vol. 17, no. 2, pp. 103–114.
Grassie, N. and Scott, G., Polymer Degradation and Stabilisation, CUP Archive, 1988.
Plesu, N., Effect of temperature on the electrochemical synthesis and properties of polyaniline films, Journal of Non-Crystalline Solids, 2010, vol. 356, no. 20, pp. 1081–1088.
Duic, L. and Grigic, S., The effect of polyaniline morphology on hydroquinone/quinone redox reaction, Electrochimica Acta, 2001, vol. 46, no. 18, pp. 2795–2803.
Ghanbari, K., Change in morphology of polyaniline/graphite composite: A fractal dimension approach, Synthetic Metals, 2006, vol. 156, no. 14, pp. 911–916.
Ehsani, A., Mahjani, M., and Jafarian, M., Electrosynthesis of poly ortho aminophenol films and nanoparticles: A comparative study, Synthetic Metals, 2012, vol. 162, no. 1, pp. 199–204.
Mahjani, M., Jafarian, M., and Ehsani, A., Poly ortho aminophenol/TiO2 nanocomposite: Electrosynthesis and electrochemical application.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2016, Vol. 52, No. 10, pp. 1048–1052.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Shayeh, J.S., Norouzi, P. & Ganjali, M.R. Effect of thickness on the capacitive behavior and stability of ultrathin polyaniline for high speed super capacitors. Russ J Electrochem 52, 933–937 (2016). https://doi.org/10.1134/S1023193516100128
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516100128