Abstract
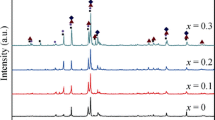
La1.8Ti0.2MgNi9–x Co x (x = 0, 0.1, 0.3, 0.5) alloys were prepared by magnetic levitation melting under argon atmosphere. The effects of Co substitution on the phase structure and the hydrogen storage properties of the alloys were investigated. The results show that LaNi5 and LaMg2Ni9 phases are contained in all experimental alloys. LaNi3 phase disappears and LaNi2 phase appears as x ≥ 0.1 and x ≥ 0.3, respectively. Electrochemical performances have been improved after Co substitution for Ni, for example, the discharge capacity and the high rate dischargeability (HRD) reach the maximum at x = 0.1, and the optimum cycling stability is obtained at x = 0.5. The positive impact of Co on the hydrogen diffusion rate in bulk enhances the HRD, but to high Co content (x ≥ 0.3), the unsatisfied hydrogen desorption capability brings relative low HRD compared with the alloy electrode at x = 0.1.
Similar content being viewed by others
References
Kohno, T., Yoshida, H., Kawashima, F., Inaba, T., Sakai, I., Yamamoto, M., and Kanda, M., J. Alloys Compd., 2000, vol. 311, p. L5.
Pan, H.G., Liu, Y.F., Gao, M.X., Lei, Y.Q., and Wang, Q.D., J. Electrochem. Soc., 2003, vol. 150, p. A5657.
Sun, X.Z., Pan, H.G., Gao, M.X., Li, R., Lin, Y., and Ma, S., Trans. Nonferr. Met. Soc. China, 2006, vol. 16, p. s834.
Dong, X.P., Yang, L.Y., Li, X.T., Wang, Q., Ma, L.H., and Lin, Y.F., J. Rare Earth., 2011, vol. 29, p. 143.
Zhang, Y.H., Li, B.W., Ren, H.P., Wu, Z.W., Dong, X.P., and Wang, X.L., Rare Met. Mat. Eng., 2009, vol. 38, p. 941.
Dong, Z.W., Ma, L.Q., Shen, X.D., Wang, L.M., Wu, Y.M., and Wang, L.D., Int. J. Hydrogen En., 2011, vol. 36, p. 893.
Guo, J., Huang, D., Li, G.X., Ma, S.Y., and Wei, W.L., Mater. Sci. Eng., Ser. B, 2006, vol. 131, p. 169.
Jiang, W.Q., Lan, Z.Q., Xu, L.Q., Li, G.X., and Guo, J., Int. J. Hydrogen En., 2009, vol. 34, p. 4827.
Zhang, X.B., Sun, D.Z., Yin, W.Y., Chai, Y.J., and Zhao, M.S., J. Solid State Electrochem., 2006, vol. 10, p. 236.
Zhao, X.L., Zhang, Y.H., Li, B.W., Ren, H.P., Dong, X.P., and Wang, X.L., J. Alloys Compd., 2008, vol. 454, p. 437.
Liu, Y.F., Pan, H.G., Gao, M.X., Li, R., and Lei, Y.Q., J. Alloys Compd., 2004, vol. 376, p. 304.
Gao, Z.J., Luo, Y.C., Lin, Z., Li, R.F., Wang, J.Y., and Kang, L., J. Solid State Electrochem., 2013, vol. 17, p. 727.
Zheng, X.P., Li, P., Islam, S.H., An, F.Q., Wang, G.Q., and Qu, X.H., Int. J. Hydrogen En., 2007, vol. 32, p. 4957.
Li, L., Qiu, F.Y., Wang, Y.J., Xu, Y.N., An, C.H., Liu, G., Jiao, L.F., and Yuan, H.T., Int. J. Hydrogen En., 2013, vol. 38, p. 3695.
Liao, B., Lei, Y.Q., Chen, L.X., Lu, G.L., Pan, H.G., and Wang, Q.D., J. Alloys Compd., 2006, vol. 415, p. 239.
Miyamura, H., Kuriyama, N., Sakai, T., Oguro, K., Kato, A., Ishikawa, H., and Iwasaki, T., J. Less-Common Met., 1991, vols. 172–174, p. 1205.
Zhang, Y.H., Zhao, D.L., Li, B.W., Zhao, X.L., Wu, Z.W., and Wang, X.L., Int. J. Hydrogen En., 2008, vol. 33, p. 1868.
Oesterreicher, H., Clinton, J., and Bittner, H., Mater. Res. Bull., 1976, vol. 11, p. 1241.
Fiorino, M.E., Opila, R.L., Konstadinidas, K., and Fang, W.C., J. Electrochem. Soc., 1996, vol. 143, p. 2422.
Choquette, Y., Ménard, H., and Brossard, L., Int. J. Hydrogen En., 1990, vol. 15, p. 21.
Bard, A.J. and Faulkner L.R., Electrochemical Methods: Fundamental and Applications, N.Y.: John Wiley & Sons, 1980.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2016, Vol. 52, No. 5, pp. 491–496.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Jiang, W., Guo, J. & Cao, S. Hydrogen storage properties of La1.8Ti0.2MgNi9–x Co x (x = 0, 0.1, 0.3, 0.5) alloys. Russ J Electrochem 52, 435–440 (2016). https://doi.org/10.1134/S1023193516050141
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516050141