Abstract
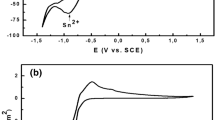
the electrochemical deposition of indium metal from InCl3 solutions was investigated. Cyclovoltammetric experiments showed that the initial hydrogen evolution reaction, observed together with the metal deposition on Pt surface, is blocked when the surface is covered by In. At large cathodic potentials, the current is diffusion-limited. The scan rate dependence of cyclovoltammograms allowed the determination of the diffusion coefficient of In3+ ions, 8.18 × 10–6 cm2/s, using the Delahay equation. The activation energy of diffusion, determined from the temperature dependence of cyclovoltammograms, is about 0.3 eV (23 kJ/mol). Chrono-amperometric experiments are consistent with the cyclovoltammetry; the In3+ diffusion coefficient determined using the Cottrell law is in good agreement with the value determined by the Randles-Ševčik equation. Moreover the use of the nucleation models developed by Scharifker and Hills showed a progressive nucleation mode. Electron microscopy observations and X-ray diffraction patterns confirmed the formation of crystalline indium deposits.
Similar content being viewed by others
References
Goetzberger, A., Hebling, C., and Schock, H.W., Mater. Sci. Eng., Ser. R, 2003, vol. 40, p. 1.
Panthani, M.G., Akhavan, V., Goodfellow, B., Schmidtke, J.P., Dunn, L., Dodabalapur, A., Barbara, P.F., and Korgel, B.A., J. Am. Chem. Soc., 2008, vol. 130, p. 16770.
Lincot, D., Thin Solid Film, 2005, vol. 487, p. 40.
Cayzac, R., Boulc’h, F., Bendahan, M., Lauque, R., and Knauth, P., Mater. Sci. Eng., Ser. B, 2009, vol. 157, p. 66.
Cayzac, R., Boulc’h, F., Hornebecq, V., Djenizian, T., Bendahan, M., Pasquinelli, M., and Knauth, P., J. Mater. Res., 2009, vol. 24, p. 3044.
Lincot, D., Guillemoles, J.F., Taunier, S., Guimard D., et al., Solar Energy, 2004, vol. 77, p. 725.
Duchatelet, A., Savidand, G., Vannier, R.N., and Lincot, D., Thin Solid Films, 2013, vol. 545, p. 94.
Valdes, M., Mollar, M., Vazquez, M., and Mari, B., J. Appl. Electrochem., 2013, vol. 43, p. 619.
Chhina, H., Campbell, S., and Kesler, O., J. Power Sources, 2006, vol. 161, p. 893.
Sharma, S. and Pollet, B.G., 2012, vol. 208, p. 96.
Granqvist, C.G. and Hultaker, A., Thin Solid films, 2002, vol. 411, p. 1.
Minami, T., Thin Solid Films, 2008, vol. 516, p. 5822.
Lupan, O., Guerin, V.M., Tiginyanu, I.M., Ursaki, V.V., Chow, L., Heinrich, H., and Pauporte, T., J. Electrochem. Photobiology, Ser. A, 2010, vol. 211, p. 65.
Miettunen, K., Halme, J., Vahermaa, P., Saukkonen, T., Toivola, M., and Lund, P., J. Electrochem. Soc., 2009, vol. 156, p. B876.
Park, Y.S., Park, H.K., Jeong, J.A., Kim, H.K., Choi, K.H., Na, S.I., and Kim, D.Y., J. Electrochem. Soc., 2009, vol. 156, p. H588.
Zhmakin, A.I., Phys. Rep., Rev. Section Phys. Lett., 2011, vol. 498, p. 189.
Hee Nam Kang, Jin-Young Lee, and Jong-Young Kim, Hydrometallurgy, 2011, vol. 110, p. 120.
Zhou Zhi-hua, Mo Hong-bing, and Zeng Dong-ming, rans. Nonferrous Met. Soc. China, 2004, vol. 14, p. 637.
Kozin, L.F., Nagibin, S.N., and Chabanenko, Y.I., High-Purity Substances, 1996, vol. 5, p. 30.
Alfantazi, A.M. and Moskalyk, R.R., Miner. Eng., 2003, vol. 16, p. 687.
Gunawardena, G., Pletcher, D., and Razaq, A.J., J. Electroanal. Chem., 1984, vol. 164, p. 363.
Walsh, F.C. and Gabe, D.R., Surf. Technol., 1979, vol. 8, p. 87.
Walsh, F.C. and Gabe, D.R., Surf. Technol., 1978, vol. 6, p. 425.
Canegallo, S., Demeneopoulos, V., Peraldo Bicelli, L., and Serravalle, G., J. Alloys Compounds, 1995, vol. 228, p. 23.
Chung, Y. and Lee, C.-W, J. Electrochem. Sci. Technol., 2012, vol. 3, p. 1.
Munoz, A.G. and Bessone, J.B., Electrochim. Acta, 1998, vol. 43, p. 1067.
Kozin, V.F. and Omelchuk, A.A., Metallurgy Rare Precious Met., 2006, vol. 2, p. 45.
Munoz, A.G. and Bessone, J.B., Electrochim. Acta, 1998, vol. 43, p. 2033.
Molodov, A.I., Markosyan, G.N., and Losev, V.V., Electrochemistry, 1973, vol. 9, p. 1368.
Dmitrenko, S.V., Molodov, A.I., and Losev, V.V., Electrochemistry, 1984, vol. 20, p. 1159.
Kozin, L.F., Sushkov, Ya.P., and Kurdyumova, T.A., Ukrain. J. Chem., 1974, vol. 40, p. 1136.
Latimer, W.M., The Oxidation States of the Elements and their Potentials in Aqueous Solutions, 2nd Ed., N.Y. Prentice-Hall, Inc., 1952.
Hepler, L.G., Hugus, Z.Z., and Latimer, W.M., J. Am. Chem. Soc., 1953, vol. 75, p. 5652.
Piercy, R. and Hampson, N.A., J. Appl. Electrochem., 1975, vol. 5, p. 1.
Timmer, B., Sluyters-Rehbach, M., and Sluyters, J.H., J. Electroanal. Chem., 1968, vol. 19, p. 73.
Scharifker, B. and Hills, G., Electrochim. Acta, 1983, vol. 28, p. 879.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2016, Vol. 52, No. 2, pp. 115–122.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Rakhymbay, G., Nauryzbayev, M.K., Burkitbayeva, B.D. et al. Electrochemical deposition of indium: nucleation mode and diffusional limitation. Russ J Electrochem 52, 99–105 (2016). https://doi.org/10.1134/S1023193516020087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516020087