Abstract
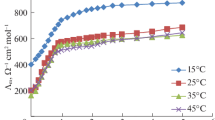
The complexation reaction between UO 2+2 cation and the macrocyclic ligand, Kryptofix 21, was studied in acetonitrile-methanol (AN–MeOH) and acetonitrile–ethylacetate (AN–EtOAc) binary solvent solutions at different temperatures using the conductometric method. In most cases, Kryptofix 21 forms a 1: 1 [M: L] complex with the UO 2+2 cation. But in some of the studied solvent systems, 1: 2 [M: L2] and also 1: 3 [M: L3] complexes are formed in solutions. The results obtained in this study show that the mechanism of the complexation process between the uranyl cation and Kryptofix 21 changes with the nature and composition of the solvent system. In the case of the binary solvent solutions (mol % AN = 50 and 60), the order of stability constant of the complex at all studying temperatures was found to be: AN–EtOAc > AN–MeOH. The values of thermodynamic quantities (ΔS °c , ΔH °c ) for the formation of (Kryptofix 21–UO2)2+ complex were obtained from temperature dependence of the stability constant of the complex using the van’t Hoff plots. The results show that the values of these parameters are influenced by the nature and composition of the mixed solvents and is most solvent systems, the 1: 1 complexation reaction between UO 2+2 and the macrocyclic ligand is athermic.
Similar content being viewed by others
References
Pedersen, C.J., J. Am. Chem. Soc., 1967, vol. 89, p. 2495.
Ekmekci, G., Uzun, D., and Somer, G., J. Memb. Sci., 2007, vol. 288, p. 36.
Chandra, S., Buschbeck, R., and Lang, H., Talanta, 2006, vol. 70, p. 1087.
Chandra, S. and Lang, H., Sens. Actuators, Ser. B, 2006, vol. 114, p. 849.
Gupta, V.K., Khayat, M.Al., and Minocha, A.K., Anal. Chim. Acta, 2005, vol. 532, p. 153.
Kim, S., Kim, H., and Noh, K.H., Talanta, 2003, vol. 61, p. 709.
Rawat, N., Mohapatra, P.K., and Lakshmi, D.S., J. Memb. Sci., 2006, vol. 275, p. 82.
Malcik, N., Tunoglu, N., and Caglar, P., Sens. Actuators, Ser. B, 1998, vol. 53, p. 204.
Seyhan, S. and Tu, Y., Tetrahedron, 2006, vol. 17, p. 1700.
Saad, B., Chong, C.C., and Ali, A.S.M., Anal. Chim. Acta, 2006, vol. 555, p. 146.
Heitzman, H., Young, B.A., and Rausch, D.J., Talanta, 2006, vol. 69, p. 527.
Nakamura, K., Nishiyama, S., and Tsuruya, S., J. Mol. Catal., 1994, vol. 93, p. 195.
Strasser, B.O., Hallenga, K., and Popov, A.I., J. Am. Chem. Soc., 1985, vol. 107, p. 789.
Loyola, V.M., Pizer, R., and Wilkins R.G., J. Am. Chem. Soc., 1977, vol. 99, p. 7185.
Rofouei, M., Ahmadalinezhah, A., and Taghdiri, M., J. Incl. Phenom. Macrocycl. Chem., 2007, vol. 58, p. 377.
Al-Mustafa, J., Hamzah, S., and Marji, D., J. Sol. Chem., 2004, vol. 30, p. 681.
Rounaghi, G.H., Rahimi Bajestani, M., and Ghaemi, A., Asian J. Chem., 2008, vol. 20, p. 299.
Rounaghi, G.H., Soleamani, A., and Sanavi, K.R., J. Incl. Phenom. Macrocycl. Chem., 2007, vol. 58, p. 43.
Rounaghi, G.H. and Gerey, N.G., Asian J. Chem., 2007, vol. 19, p. 929.
Rounaghi, G.H., Masroornia, M., and Ghaemi A., Asian J. Chem., 2007, vol. 19, p. 1679.
Rounaghi, G.H. and Sanavi, R., Pol. J. Chem., 2006, vol. 80, p. 719.
Rounaghi, G.H., Sanavi Khoshnood, R., and Arbab Zavvar, M.H., J. Incl. Phenom. Macrocycl. Chem., 2006, vol. 54, p. 247.
Seller, R.M., Radiat. Phys. Chem., 1983, vol. 21, p. 295.
Rounaghi, G.H., Mohammad Zade Kakhki, R., J. Incl. Phenom. Macrocycl. Chem., 2009, vol. 63, p. 117.
Ansari Fard, M., Rounaghi, G.H., and Chamsaz, M., J. Incl. Phenom. Macrocycl. Chem., 2009, vol. 64, p. 49.
Rounaghi, G.H., Nazari, E., and Ghaemi, A., J. Coord. Chem., 2010, vol. 63, p. 2349.
Ansari Fard, M., Rounaghi, G.H., and Chamsaz, M., Asian J. Chem., 2009, vol. 21, p. 2799.
Genplot Computer Graphic Service, U.S.A., 1989.
Gutmann, V., N.Y.: Plenum press, 1978.
Rounaghi, G.H. and Popov, A.I., Polyhedron, 1986, vol. 5, p. 1935.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2015, Vol. 51, No. 8, pp. 856–862.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Nasiri, M., Rounaghi, G.H. Study of complex formation between Kryptofix 21 and UO 2+2 cation in some binary mixed non-aqueous solutions. Russ J Electrochem 51, 758–763 (2015). https://doi.org/10.1134/S102319351508008X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102319351508008X