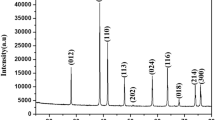
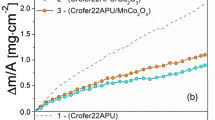
Abstract
The structure and properties of Fe–Mo alloys is studied. It is shown that the iron–molybdenum alloys formed in the electrolysis are highly hydrogenated, and the content of hydrogen in them increases with increasing atomic fraction of molybdenum in the cathodic deposit. The kinetics of hydrogen evolution reaction in the alkaline solutions on the obtained materials is studied.
Similar content being viewed by others
References
Elezovic, N.R., Jovic, V.D., and Krstajic, N.V., Electrochim. Acta, 2005, vol. 50, p. 5594.
Jaksic, M.M., Electrochim. Acta, 1984, vol. 29, p. 1539.
Jaksic, J.M., Radimilovic, V.R., Krstajic, N.V., Lacnjevac, C.M., and Jaksic, M.M., Macedonian J. Chem. and Chem. Eng., 2011, vol. 30, p. 3.
Trasatti, S., Adv. Electrochem. Sci. Eng., 1992, vol. 2, p. 1.
Petrii, O.A. and Tsirlina, G.A., Electrochim. Acta, 1994, vol. 39, p. 1739.
Li, Z., Shen, L.-T., Huang, W., and Xie, K.-C., J. Environ. Sci., 2007, vol. 19, p. 1516.
Pavanati, H.C., Lourenco, J.M., Maliska, A.M., Klein, A.N., and Muzart, J.L.R., Appl. Surf. Sci., 2007, vol. 253, p. 9105.
Fan, C., Piron, D.L., Sled, A., and Paradis, P., J. Electrochem. Soc., 1994, vol. 141, p. 382.
Hashimoto, K., Sasaki, T., Meguru, S., and Asami, K., Mater. Sci. Eng., A, 2004, vols. 375-377, p. 942.
Kuznetsov, V.V., Golyanin, K.E., Lyashenko, S.E., and Lyakhov, B.F., Gal’vanotekh. Obrab. Poverkhn., 2013, vol. 21, no. 4, p. 18.
Charlot, G., Les Methodes de la Chimie Analytique. Analyse Quantitative Minerale, Paris: Masson, 1961, part 2.
Moulder, J.F., Stickle, W.F., Sobol, P.E., and Bomben, K.D., Handbook of X-Ray Photoelectron Spectroscopy, Eden Prairie (MN, USA): Perkin-Elmer, 1992.
Platonov, B.M., Urin, O.V., and Polukarov, Yu.M., Elektrokhimiya, 1985, vol. 21, p. 1699.
Karyakin, Yu.V. and Angelov, I.I., Chistye khimicheskie veshchestva (Pure Chemical Substances), Moscow: Gos. Nauch.-Tekhn. Izd. Khim. Lit., 1955, p. 376.
Kuz’menko, B.V. and Krishtalik, L.I., Elektrokhimiya, 1973, vol. 9, p. 130.
Podlaha, E.J. and Landolt, D., J. Electrochem. Soc., 1997, vol. 144, p. 1672.
Kuznetsov, V.V. and Pshenichkina, T.V., Russ. J. Electrochem., 2010, vol. 46, p. 401.
Protsenko, V.S., Gordienko, V.O., and Danilov, F.I., Electrochem. Commun., 2012, vol. 17, p. 85.
Edigaryan, A.A., Safonov, V.A., Lubnin, E.N., Vykhodtseva, L.N., Chusova, G.E., and Polukarov, Yu.M., Electrochim. Acta, 2002, vol. 47, p. 2775.
Kravtsov, V.I., Ravnovesie i kinetika elektrodnykh reaktsii kompleksov metallov (Equilibrium and Kinetics of Electrode Reactions of Metal Complexes), Leningrad: Khimiya, 1985.
Basolo, F. and Pearson, R.G., Mechanisms of Inorganic Reactions: Study of Metal Complexes in Solution, Wiley, 1967.
Scardi, P., Leoni, M., and Delhez, R., J. Appl. Crystallogr., 2004, vol. 37, p. 381.
Idczak, R., Konieczny, R., and Chojcan, J., Hyperfine Interact., 2012, vol. 208, p. 1.
Marcus, H.L. and Schwartz, L.H., Phys. Rev., 1967, vol. 162, p. 259.
Bokshtein, B.S, Voitkovskii, Yu.B., Latash, E.G., Nadzharov, A.Yu., and Nikol’skii, G.S., Metalloved. Term. Obrab. Met., 1974, no. 6, p. 64.
Spravochnik po elektrokhimii (Handbook of Electro-chemistry), Sukhotin, A.M., Ed., Leningrad: Chemistry, 1981.
Encyclopedia of Electrochemistry of Elements, vol. 9}, Part A: Hg, Fe, H, New York: Marcel Dekker, 1982
Damaskin, B.B. and Petrii, O.A., Vvedenie v elek-trokhimicheskuyu kinetiku (Introduction to Electrochemical Kinetics), Moscow: Vyssh. Shkola, 1983.
Krishtalik, L.I., Elektrodnye reaktsii. Mekhanizm elementarnogo akta (Electrode Reactions: Mechanism of Elementary Act), Moscow: Nauka, 1979.
Shriver, D. and Atkins, P.W., Inorganic Chemistry, New York: Freeman, 1999.
Quaino, P., Arce, M.D., Santos, E., and Schmicker, W., Int. J. Hydrogen Energy, 2012, vol. 37, p. 14796.
Prikladnaya elektrokhimiya (Applied Electrochemistry), Tomilov, A.P., Ed., Moscow: Khimiya, 1984.
Luo, B., Ren, B., Xu, Y., and Zheng, Ya., Rare Metals, 2007, vol. 26, p. 205.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Kuznetsov, K.E. Golyanin, Yu.Sh. Ladygina, T.V. Pshenichkina, B.F. Lyakhov, K.V. Pokholok, 2015, published in Elektrokhimiya, 2015, Vol. 51, No. 8, pp. 846–855.
Rights and permissions
About this article
Cite this article
Kuznetsov, V.V., Golyanin, K.E., Ladygina, Y.S. et al. Electrodeposition of iron–molybdenum alloy from ammonium–citrate solutions and properties of produced materials. Russ J Electrochem 51, 748–757 (2015). https://doi.org/10.1134/S1023193515080066
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193515080066