Abstract
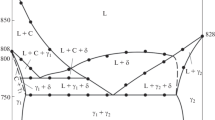
Solid solutions of Sm2S3 and Tm2S3 based on BaSm2S4 (the structural type of CaFe2O4) are obtained. The samples are studied using microprobe analysis and the XRD technique. The length of the solid solution region is determined. The type of ion conductivity and thermodynamics of dissolution of the binary sulfides of Sm2S3 and Tm2S3 in BaSm2S4 are studied. Partial molar enthalpies of dissolution of binary sulfides in basic thiosamarate are determined. A possible mechanism of defect formation is suggested.
Similar content being viewed by others
References
Kalinina, L.A., Shirokova, G.I., Murin, I.V., Ushakova, Yu.N., Fominykh, E.G., and Lyalina, M.Yu., Zh. Prikl. Khim., 2000, vol. 73, no. 8, p. 1324.
Ushakova, Yu.N., Kalinina, L.A., Ananchenko, B.A., Yurlov, I.S., Shirokova, G.I., and Fominykh, E.G., Fiz. Khim. Stekla, 2009, vol. 35, no. 3, p. 429.
Ushakova, Yu.N., Kalinina, L.A., Yurkov, I.S., Bauderina, T.V., and Murin, I.V., Russ. J. Electrochem., 2009, vol. 45, p. 677.
Chebotin, V.N. and Obrosov, V.P., Tr. Inst. Elektrokhim., Ural. Fil., Akad. Nauk SSSR, 1972, no. 18, p. 151.
Chebotin, V.N., Fizicheskaya khimiya tverdogo tela (Physical Chemistry of Solids), Moscow: Khimiya, 1982.
Kalinina, L.A., Ushakova, Yu.N., Medvedeva, O.V., Shirokova, G.I., and Fominykh, E.G., Russ. J. Phys. Chem., 2006, vol. 80, no. 11, p. 1731.
Gerasimov, L.I., Kutsenok, I.V., and Geidrikh, L.I., Dokl. Akad. Nauk SSSR, 1979, vol. 244, no. 3, p. 633.
Andreev, O.V., Parshukov, N.N., and Bamburov, V.G., Zh. Neorg. Khim., 1998, vol. 43, no. 5, p. 853.
Mikhailichenko, N.V., Kalinina, L.A., Ushakova, Yu.N., Shyrokova, G.I., and Tokareva, T.V., Russ. J. Electrochem., 2011, vol. 47, p. 556.
Chebotin, V.N. and Perfil’ev, M.V., Elektrokhimiya tverdykh elektrolitov (Electrochemistry of Solid Electrolytes), Moscow, 1978.
Tret’yakov, Yu.D., Khimiya nestekhiometricheskikh okislov (Chemistry of Nonstoichiometric Oxides), Moscow: Izd-vo MGU, 1974.
Tret’yakov, Yu.D., Tverdofaznye reaktsii (Solid-Phase Reactions), Moscow: Khimiya, 1978.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.V. Mikhailichenko, L.A. Kalinina, Yu.N. Ushakova, I.V. Murin, 2013, published in Elektrokhimiya, 2013, Vol. 49, No. 8, pp. 865–871.
Published on the basis of the lecture in XI Meeting “Fundamental Problems of Solid State Ionics”, Chernogolovka, 2012.
Rights and permissions
About this article
Cite this article
Mikhailichenko, T.V., Kalinina, L.A., Ushakova, Y.N. et al. Ion conductivity type and thermodynamics of dissolution of binary sulfides Sm2S3 and Tm2S3 in BaSm2S4 . Russ J Electrochem 49, 776–782 (2013). https://doi.org/10.1134/S1023193513080132
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193513080132