Abstract
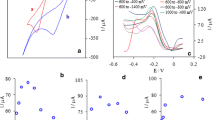
Cyclic voltammetry (CV), double-potential step chronocoulometry (DPSCC), and electrochemical impedance spectroscopy (EIS) techniques have been performed to study the effects of abrasive particles on the electrochemical reaction of adrenaline at glassy carbon electrode (GCE) and platinum electrode in 0.5 mol/L H2SO4 solution. For the electrochemical reaction of adrenaline, it was shown that abrasive particles have a more marked electrocatalytic effect at GCE compared to that at platinum electrode. The electrocatalytic effect of SiC coated GCE is more obvious comparing to that of Al2O3 coated GCE. With the coarse degree of the abrasive paper increasing, the peak current (i p) increases significantly and the peak-to-peak potential separation (ΔE p) changes a little at the pretreated GCE. The electron transfer process of adrenaline at the different pretreated GCE is controlled by the diffusion in this system.
Similar content being viewed by others
References
Hawley, M.D., Tatawawadi, S.V., Piekarski, S., and Adams, R.N., J. Am. Chem. Soc., 1967, vol. 89, p. 447.
Fike, R.E. and Curran, D.J., Anal. Chem., 1977, vol. 49, p. 1205.
Zhang, L.K., Yang, Y.J., Liang, B.A., and Hu, S.S., Russ. J. Electrochem., 2011, vol. 47, p. 799.
Latham, R.J., Linford, R.G., and Schlindwein, W.S., Ionics, 2003, vol. 9, p. 41.
Zak, J. and Kuwana, T., J. Am. Chem. Soc., 1982, vol. 104, p. 5514.
Dong, S.J. and Kuwana, T., J. Electrochem. Soc., 1984, vol. 131, p. 813.
Zhang, H., Gui, X.Q., Xu, Y., and Jin, B.K., Chinese Chem. Lett., 2002, vol. 13, p. 153.
Zak, J. and Kuwana, T., J. Electroanal. Chem., 1983, vol. 150, p. 645.
Ghavamia, R., Salimia, A., and Navaee, A., Biosens. Bioelectron., 2011, vol. 26, p. 3864.
Wu, W.C., Chang, H.W., and Tsai, Y.C., Chem. Commun., 2011, vol. 47, p. 6458.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, 2nd edition, New York: John Wiley & Sons, Inc., 2001.
Yu, Z.Y., Guo, T.D., and Qin, M., Anal. Chem., 1994, vol. 66, p. 497.
Boukamp, B.A., Equivalent Circuits: Users Manual, 2nd edition, University of Twente, The Netherlands, 1993
McDermott, M.T., Kneten, K., and McCreery, R.L., J. Phys. Chem., 1992, vol. 96, p. 3124.
Chen, P.H. and McCreery, R.L., Anal. Chem., 1996, vol. 68, p. 3958.
Rusling, J.F., Anal. Chem., 1984, vol. 56, p. 575.
Nagaoka, T. and Yoshino, T., Anal. Chem., 1986, vol. 58, p. 1037.
Ji, H.M. and Wang, E.K., Acta Chim. Sinica, 1989, vol. 47, p. 867.
Corona-Avendaño, S., Alarcón-ángeles, G., Ramírez-Silva, M. T., Rosquete-Pina, G., Romero-Romo, M., and Palomar-Pardavé, M., J. Electroanal. Chem., 2007, vol. 609, p. 17.
Zheng, D.H., Lu, T.H., Zhang, C.Z., and Li, G.Z., Acta Phys.-Chim., 1997, vol. 13, p. 797.
Brett, C.M.A. and Brett, A.M.O., in Electrochemistry: Principles, Methods and Applications, Oxford: Oxford University Press, 1993.
Wu, X., Mu, L., and Zhang, W., J. Electroanal. Chem., 1993, vol. 352, p. 295.
Pound, B.G., Electrochim. Acta, 1993, vol. 38, p. 2021.
Ma, H., Chen, S., Cheng, X., Chen, X., Li, G., and Yang, X., J. Serb. Chem. Soc., 1997, vol. 62, p.1201.
Bisquert, J., Garcia-Belmonte, G., Fabregat-Santiago, F., and Bueno, P.R., J. Electroanal. Chem., 1999, vol. 475, p. 152.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2014, Vol. 50, No. 1, pp. 89–96.
The aricle is published in the original.
Rights and permissions
About this article
Cite this article
Yuan, ZD. The effects of abrasive particles on the electrochemical behavior of adrenaline at different electrodes. Russ J Electrochem 50, 80–86 (2014). https://doi.org/10.1134/S1023193513060074
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193513060074