Abstract
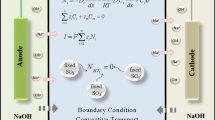
In an unforced flowing NaCl solution in bulk, gravitational or electro convection supplies ions from bulk toward the membrane surface through a boundary layer. In a boundary layer formed on an anion exchange membrane, the convection converts to migration and diffusion and carries an electric current. In a boundary layer formed on a cation exchange membrane, the convection converts to migration and carry an electric current. In a forced flowing solution in bulk, the boundary layer thickness is reduced and gravitation or electro convection is disappeared. An electric current is carried by diffusion and migration on the anion exchange membrane and by migration on the cation exchange membrane. Ion transport in a boundary layer on the cation exchange membrane immersed in a NaCl solution is more restricted comparing to the phenomenon on the anion exchange membrane. This is due to lower counter-ion mobility in the boundary layer and the restricted water dissociation reaction in the membrane. The water dissociation reaction is generated in an ion exchange membrane and promoted due to the increased forward reaction rate constant. However, the current efficiency for the water dissociation reaction is generally low. The intensity of the water dissociation is more suppressed in the strong acid cation exchange membrane comparing to the phenomenon in the strong base anion exchange membrane due to lower forward reaction rate constant in the cation exchange membrane. In the strong acid cation exchange membrane, the intensity of electric potential is larger than the values in the strong base anion exchange membrane. Accordingly, the stronger repulsive force is developed between ion exchange groups (SO •3 groups) and co-ions (OH− ions) in the cation exchange membrane, and the water dissociation reaction is suppressed. In the strong base anion exchange membrane, the repulsive force between ion exchange groups (N+(CH3)3 groups) and co-ions (H+ ions) is relatively low, and the water dissociation reaction is not suppressed. Violent water dissociation is generated in metallic hydroxides precipitated on the desalting surface of the cation exchange membrane. This phenomenon is caused by a catalytic effect of metallic hydroxides. Such violent water dissociation does not occur on the anion exchange membrane.
Similar content being viewed by others
References
Peer, A.M., Discus, Communication in the Membrane Phenomena Special Issue, Faraday Sci., 1956, vol. 21, p. 124.
Cowan, D.A. and Brawn, J.H., Effect of Turbulence on Limiting Current in Electrodialysis Cells, Ind. Eng. Chem., 1959, vol. 51, pp. 1445–1448.
Spiegler, K.S., Polarization at Ion Exchange Membrane-Solution Interface, Desalination, 1971, vol. 9, pp. 365–385.
Krol, J.I., Wessling, M., and Strathmann, H., Concentration Polarization with Ion Exchange Membrane: Current-Voltage Curves and Water Dissociation, J. Membr. Sci., 1999, vol. 162, pp. 145–454.
Valerdi-Prez, R. and Ibannez-Mengual, J.A., Current-Voltage Curves for an Electrodialysis Reversal Pilot Plant: Determination of Limiting Currents, Desalination, 2001, vol. 141, pp. 23–37.
Kitamoto, A. and Takashima, Y., Studies on Electrodialysis, Maximum Attainable Concentration, Limiting Current Density and on Energy Efficiency in Electrodialysis Using Ion-Exchange Membranes, J. Chem. Eng. Jpn., 1968, vol. 32, pp. 74–82.
Miyoshi, H., Fukumoto, T., and Kataoka, T., AMethod for Estimating the Limiting Current Density in Electrodialysis, Sep. Sci. Technol., 1988, vol. 23, pp. 585–600.
Huang, T.C. and Yu, I.Y., Correlation of Ionic Transfer Rate in Electrodialysis under Limiting Current Density Conditions, J. Membr. Sci., 1988, vol. 35, pp. 193–206.
Rubinstein, I. and Shtilman, L., Voltage Against Current Curves of Cation-Exchange Membranes, J. Chem. Soc., Faraday Trans. II, 1979, vol. 75, pp. 231–246.
Rubinstein, I., Mechanism for an Electrodiffusion Instability in Concentration Polarization, J. Chem. Soc., Faraday Trans II, 1981, vol. 77, pp. 1595–1609.
Frilette, V.J., Electrogravitational Transport at Synthetic Ion Exchange Membrane Surface, J. Phys. Chem., 1957, vol. 61, pp. 168–174.
Gavish, B. and Lifson, S., Membrane Polarization at High Current Densities, J. Chem. Soc., Faraday Trans. I, 1979, vol. 75, pp. 463–472.
Zabolotsky, V.I., Nikonenko, V.V., and Pismenskaya, N.D., On the Role of Gravitational Convection in the Transfer Enhancement of Salt Ions in the Course of Dilute Solution Electrodialysis, J. Membr. Sci., 1996, vol. 119, pp. 171–181.
Rubinstein, I., Staude, E., and Kedem, O., Role of the Membrane Surface in Concentration Polarization at Ion-Exchange Membrane, Desalination, 1988, vol. 69, pp. 101–104.
Zabolotsky, V.I., Shel’deshov, N.V., and Gnusin, N.P., Dissociation of Water Molecules in System with Ion-Exchange Membranes, Russ. Chem. Rev., 1988, vol. 57, pp. 801–808.
Nikonenko, V.V., Pismenskaya, N.D., Belova, E.I., Sistat, P., Huguet, P., Pourcelly, G., and Larchet, C., Intensive Current Transfer in Membrane System: Modeling, Mechanisms and Application in Electrodialysis, Adv. Colloid Interface Sci., 2010, vol. 160, pp. 101–123.
Rubinstein, S.M., Manukyan, G., Staicu, A., Rubinstein, I., Zaltzman, B., Lammertink, R.G.H., Mugele, F., and Wessling, M., Direct Observation of a Nonequilibrium Electro-Osmotic Instability, Phys. Rev. Lett., PRL, 2008, vol. 101, p. 236101.
Yossifon, G. and Chang, H.C., Selection of Nonequilibrium Overlimiting Currents: Universal Depletion Layer Formation Dynamics and Vortex Instability, Phys. Rev. Lett., 2008, vol. 101, p. 254501.
Shaposhnik, V.A., Vasil’eva, V.I., and Grigorchuk, O.V., The Interferometric Investigations of Electromembrane Processes, Adv. Colloid Interface Sci., 2008, vol. 139, pp. 74–82.
Green, M.E. and Yafuso, M., A Study of the Noise Generated During Ion Transport Across Membranes, J. Phys. Chem., 1968, vol. 72, pp. 4072–4078.
Krol, J.J., Wessling, M., and Strathmann, H., Chronopotentiometry and Overlimiting Ion Transport through Monomer Ion Exchange Membranes, J. Membr. Sci., 1999, vol. 162, pp. 155–164.
Lifson, S., Gavish, B., and Reich, S., Flicker Noise of Ion-Exchange Membranes and Turbulent Convection in the Depleted Layer, Biophys. Struct. Mechanism, 1978, vol. 4, pp. 53–65.
Li, Q., Fang, Y., and Green, M.E., Turbulent Light-Scattering Fluctuation Spectra near a Cation Electrodialysis Membrane, J. Colloid Interface Sci., 1983, vol. 91, pp. 412–417.
Kressman, T.R.E. and Tye, F.L., The Effect of Current Density on the Transport of Ions through Ion-Selective Membranes, Discuss. Faraday Soc., 1956, vol. 21, pp. 185–192.
Frilette, V.I., Preparation and Characterization of Bipolar Ion-Exchange Membranes, J. Phys. Chem., 1956, vol. 60, pp. 435–439.
Rosenberg, N.W. and Tirrel, C.E., Limiting Currents in Membrane Cells, Ind. Eng. Chem., 1957, vol. 49, pp. 780–784.
Rubinstein, I., A Diffusion Model of “Water Splitting” in Electrodialysis, J. Phys. Chem., 1977, vol. 81, pp. 1431–1436.
Patel, R.D., Lang, K.C., and Miller, I.F., Polarization in Ion-Exchange Membrane Electrodialysis, Ind. Eng. Fundam., 1977, vol. 16, pp. 340–348.
Wien, M., Uber die abweichungen der electrolyte vom Ohmschen gesetz, Phys. Zeits, 1928, vol. 29, pp. 751–755.
Onsager, L., Deviation from Ohm’s Law in Weal Electrolyte, J. Chem. Phys., 1934, vol. 2, pp. 599–615.
Simons, R., Electric Field Effects on Proton Transfer between Ionizable Groups and Water in Ion Exchange Membranes, Electrochim. Acta, 1984, vol. 29, pp. 151–158.
Simons, R., Water Splitting in Ion Exchange Membranes, Electrochim. Acta, 1985, vol. 30, pp. 275–282.
Tanaka, Y., Concentration Polarization in Ion-Exchange Membrane Electrodialysis—the Events Arising in a Flowing Solution in a Desalting Cell, J. Membr. Sci., 2003, vol. 216, pp. 149–164.
Tanaka, Y., Concentration Polarization on Ion-Exchange Membrane Electrodialysis. The Events Arising in an Unforced Flowing Solution in a Desalting Cell, J. Membr. Sci., 2004, vol. 244, pp. 1–16.
Tanaka, Y., Water Dissociation Reaction Generated in an Ion Exchange Membrane, J. Membr. Sci., 2010, vol. 350, pp. 347–360.
Tanaka, Y., Acceleration of Water Dissociation Generated in an Ion Exchange Membrane, J. Membr. Sci., 2007, vol. 303, pp. 234–243.
Takemoto, N., The Concentration Distribution in the Interfacial Layer at Desalting Side in Ion Exchange Membrane Electyrodialysis, J. Chem. Soc. Jpn., 1972, vol. 1972, pp. 2053–2058.
Tanaka, Y., Irreversible Thermodynamics and Overall Mass Transport in Ion-Exchange Membrane Electrodialysis, J. Membr. Sci., 2006, vol. 281, pp. 517–531.
Cowan, D.A. and Brown, J.H., Effect of Turbulence on Limiting Current in Electro-Dialysis Cells, Ind. Eng. Chem., 1959, vol. 51, pp. 1445–1448.
Nernst, W., Zur kinetik der in losung befindlichen korper, Z. Phys. Chem., 1888, vol. 2, pp. 613–637.
Tanaka, Y., Concentration Polarization and Dissociation of Water in the Ion Exchange Membrane Electrodialysis, J. Electrochem., Jpn., 1974, vol. 42, pp. 450–546.
Eigen, M., Method for Investigation of Ionic Reaction in Aqueous Solutions with Half-Times as Short as 10−9 s, Application to Neutralization and Hydrolysis Reaction, Discuss. Faraday Soc., 1954, vol. 17, pp. 194–205.
Tanaka, Y., Ion Exchange Membrane: Fundamentals and Application, in Membrane Science and Technology Series, Amsterdam: Elsevier, 2007, vol. 12.
Tanaka, Y., Matsuda, S., Sato, Y., and Seno, M., Concentration Polarization and Dissociation of Water in Ion Exchange Membrane Electrodialysis, III: The Effects of Electrolytes on the Dissociation of Water, J. Electrochem. Jpn., 1982, vol. 50, pp. 667–672.
Tanaka, Y. and Seno, M., The Concentration Polarization and Dissociation of Water in Ion Exchange Membrane Electrodialysis, V: The Acceleration of Ionic Transport on the Membrane Surface, J. Electrochem., Jpn., 1983, vol. 51, pp. 267–271.
Timashev, S.F. and Kirganova, E.V., Mechanism of the Electrolytic Decomposition of Water Molecules in Bipolar Ion-Exchange Membranes, Sov. Electrochem., 1982, vol. 17, pp. 366–369.
Mafe, S., Ramirez, P., and Alcaraz, A., Electric Field Assisted Proton Transfer and Water Dissociation at the Junction of a Fixed-Charge Bipolar Membrane, Chem. Phys. Lett., 1998, vol. 294, pp. 406–412.
Donnan, F.G., The Theory of Membrane Equilibria, Chem. Rev., 1924, vol. 1, pp. 73–90.
Kolyubin, A.V., Maksimychev, A.V., and Timashev, S.F., The Use of Flicker-Noise Spectroscopy for Studying the Mechanism of the Overlimiting Current in a System with Cation-Exchange Membrane, Russ. J. Chem., 1966, vol. 32, pp. 206–213.
Helfferich, H., Ion Exchange, New York: McGraw-Hill, 1962, p. 86.
Kang, M.S., Choi, Y.J., and Moon, S.H., Effects of Inorganic Substances on Water Splitting in Ion-Exchange Membranes, II: Optimal Contents of Inorganic Substances in Preparing Bipolar Membranes, J. Colloid Interface Sci., 2004, vol. 273, pp. 533–539.
Oda, Y. and Yawataya, T., Neutrality-Disturbance Phenomenon of Membrane-Solution Systems, Desalination, 1968, vol. 5, pp. 129–138.
Ganych, V.V., Zabolotskii, V.I., and Shel’deshov, N.V., Electrolytic Dissociation of Water Molecules in Systems Comprising Solutions and MA-40 Anion-Exchange Membranes Modified with Transition Metal Ions, Sov. Electrochem., 1992, vol. 28, pp. 1138–1143 [Elektrokhimiya (Engl. Transl.), 1992, vol. 28, pp. 1390–1396].
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2012, Vol. 48, No. 7, pp. 739–755.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Tanaka, Y. Mass transport in a boundary layer and in an ion exchange membrane: Mechanism of concentration polarization and water dissociation. Russ J Electrochem 48, 665–681 (2012). https://doi.org/10.1134/S1023193512060122
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193512060122