Abstract
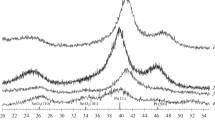
The effect of the nature of transient metal and chemical treatment of binary cathodic PtM/C (M = Co, Ni, Cr) catalysts, which were prepared by high-temperature synthesis, on their structure, surface segregation, and characteristic properties (activity and stability) is studied. It is shown that, in the course of treatment in 0.5 M H2SO4 at the elevated temperature (60°C), the surface of nanoparticles becomes enriched in platinum with the formation of core-shell structures. The PtCo/C catalyst is the most efficient one. In this case, a compromise between the corrosion resistance and electrocatalytic activity is reached due to a higher, as compared with PtNi/C and PtCr/C, degree of alloy formation and enriching of surface in platinum in the course of corrosive attack. Thereby, the properties of platinum on the core surface change as a result of a pronounced ligand effect of the core. Thus, depending on the nature of transient metal, the binary cathodic PtM/C catalysts differ in their activity and stability, which depend on the degree of alloy formation and a possibility of formation of core-shell structure as a result of surface segregation in the course of synthesis and chemical treatment.
Similar content being viewed by others
References
Mukerjee, S., Srinivasan, S., Soriaga, M.P., and McBreen, J., J. Phys. Chem., 1995, vol. 99, p. 4577.
Thompset, D., in Handbook of Fuel Cells. Fundamental and Applications, Vielstich, W., Lamm, A., and Gasteiger, H.A., Eds., Chichester, UK: Wiley, 2003, vol. 3, ch. 37, p. 467.
Antolini, E., Salgaro, J.R.C., and Gonzalez, E.R., J. Power Sources, 2006, vol. 160, p. 957.
Stamenkovic, V., Schmidt, T.J., Ross, P.N., and Markovic, N.M., J. Phys. Chem. B, 2002, vol. 106, p. 11970.
Colon-Mercado, H.R. and Popov, B.N., J. Power Sources, 2006, vol. 155, p. 253.
Koh, S., Chengfei, Y., Mani, P., Srivastava, R., and Strasser P, J. Power Sources, 2007, vol. 172, p. 50.
Bogdanovskaya, V.A., Tarasevich, M.R., Kuznetsova, L.N., and Radina, M.V., Zh. Fiz. Khim., 2009, vol. 83, no. 12, p. 2244 [Russ. J. Phys. Chem. A, (Engl. Transl.), vol. 83, no. 12, p. 2045].
Nilekar, A.U., Xu, Y., and Zhang, J., Top. Catal., 2007, vol. 46, p. 276.
Adzic, R.R., Zhang, J., Sasaki, K., Vukmirovic, M.B., Shao, M., Wang, J.X., Nilekar, A.U., Mavrikakis, M., Valerio, J.A., and Uribe, F., Top. Catal., 2007, vol. 46, p. 249.
Debe, M.K., in Handbook of Fuel Cells. Fundamental and Applications, Vielstich, W., Lamm, A., and Gasteiger, H.A., Eds., Chichester, UK: Wiley, 2003, vol. 3, ch. 45, p. 576.
Moffat, T.P., Mallett, J.J., and Hwang, S.-M., J. Electrochem. Soc., 2009, vol. 156, p. B238.
Yuyan Shao, Jiehe Sui, Geping Yin, and Yuinzhi Gao, Appl. Catal. B: Environmental, 2008, vol. 79, p. 89.
Koh, S. and Strasser, P., J. Am. Chem. Soc., 2007, vol. 129, p. 12624.
Mani, P., Srivastava, R., and Strasser, P., J. Phys. Chem. C, 2008, vol. 112, p. 2770.
Zhang, J., Lima, F.H.B., Shao, M.H., Sasaki, K., Wang, X., Hanson, J., and Adzic, R.R., J. Phys. Chem. B, 2005, vol. 109, p. 22701.
Ross, P.N, in Electrocatalysis, Lipkowski, J. and Ross, P.N., Eds., New York: Wiley, 1998, p. 43.
Watanabe, M., Tsurumi, K., Mizukami, T., Nakamura, T., and Stonehart, P., J. Electrochem. Soc., 1994, vol. 141, p. 2659.
Kitchin, J.R., Norskov, J.K., Barteau, M.A., and Chen, J.G., J. Chem. Phys., 2004, vol. 120, p. 10240.
Greeley, J., Norskov, J.K., and Mavrikakis, M., Fnn. Rev. Phys. Chem, 2002, vol. 53, p. 319.
Travitsky, N., Ripenbein, T., Golodnitsky, D., Rosenberg, Y., Burshtein, L., and Peled, E., J. Power Sources, 2006, vol. 161, p. 782.
Guterman, A.V., Pakhomova, E.B., Guterman, V.E., Kabirov, Yu.V., and Grigor’ev, V.P., Neorg. Mater., 2009, vol. 45, p. 829.
Gasteiger, H.A., Kocha, S.S., Sompalli, B., and Wagner, F.T., Appl. Catalysis B: Environmental, 2005, vol. 56, p. 9.
Paulus, U.A., Wokaun, A., Scherer, G.G., Schmidt, T.J., Stamenkovic, V., Markovic, M., and Ross, P.N., Electrochim. Acta, 2002, vol. 47, p. 3787.
Tarasevich, M.R., Khrushcheva, E.I., and Filinovskii, V.Yu., Vrashchayushchiisya diskovyi elektrod s kol’tsom (Ring-Disk Rotating Electrode), Moscow: Nauka, 1987.
Tarasevich, M.R., Chalykh, A.E., Bogdanovskaya, V.A., Kuznetsova, L.N., Kapustina, N.A., Efremov, B.N., Ehrenburg, M.R., and Reznikova, L.A., Electrochim. Acta, 2006, vol. 51, p. 4455.
Safonov, V.A., Lapa, A.S., Mansurov, G.N., and Petrii, O.A., Elektrokhimiya, 1980, vol. 16, p. 439.
Bogdanovskaya, V.A., Tarasevich, M.R., Kuznetsova, L.N., Zhutaeva, G.V., and Lozovaya, O.V., Elektrokhimiya, 2010, vol. 46, no. 8 [Russ. J. Electrochem. (Engl. Transl.), vol. 46, no. 8].
Mitsushima, S., Koizumi, Y., Uzuka, S., and Ota, K.-I., Electrochim. Acta, 2008, vol. 54, p. 455.
Umeda, M., Maruta, T., Inoue, M., and Nakazava, A., J. Phys. Chem. C, 2008, vol. 112, p. 18098.
Tarasevich, M.R., Bogdanovskaya, V.A., Lubnin, E.N., and Reznikova, L.A., Koroziya: Materialy, Zashchita, 2006, no. 10, p. 22.
Xingwen, Yu. and Siyu, Ye., J. Power Sources, 2007, vol. 172, p. 145.
Bonakdarpour, A., Lake, K., Stevens, K., and Dahn, J.R., J. Electrochem. Soc., 2008, vol. 155, p. B108.
Tarasevich, M.R, Sadkovsky, A, and Yeager, E, in Comprehensive Treatise of Electrochemistry, Conway, B.E., Bockris, J.O., Yeager, E., Kahn, S.U.M., and White, R.E., Eds., New York: Plenum, 1983, p. 301.
Šepa, D.B., Vojnović, M.V., and Damjanović, A., Electrochim. Acta, 1981, vol. 26, p. 781.
Stamenkovic, V., Schmidt, T.J., Ross, P.N., and Markovic, N.M., J. Electroanal. Chem., 2003, vol. 554–555, p. 191.
Wei, Z., Guo, H., and Tang, Z., J. Power Sources, 1996, vol. 62, p. 233.
Salgado, J.R.C., Antolini, E., and Gonzalez, E.R., J. Phys. Chem. B, 2004, vol. 108, p. 17767.
Colon-Mercado, H.R., Kim, H., and Popov, B.N., Electrochem. Commun, 2004, vol. 6, p. 795.
Sukhotin, A.M. and Lisovaya, E.V., in Itogi Nauki Tekh., Ser.: Korroz. Zashch. Korroz., Moscow: VINITI, 1986, vol. 12, p. 61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.A. Bogdanovskaya, M.R. Tarasevich, L.A. Reznikova, L.N. Kuznetsova, 2010, published in Elektrokhimiya, 2010, Vol. 46, No. 9, pp. 1077–1087.
Rights and permissions
About this article
Cite this article
Bogdanovskaya, V.A., Tarasevich, M.R., Reznikova, L.A. et al. Composition, surface segregation, and electrochemical properties of binary PtM/C (M = Co, Ni, Cr) catalysts. Russ J Electrochem 46, 1011–1020 (2010). https://doi.org/10.1134/S1023193510090077
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193510090077