Abstract
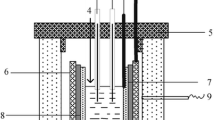
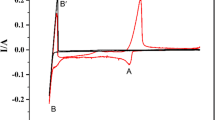
The effect of solvent salt cation, temperature of electrolysis, and cathodic deposition potential to basic characteristics of uranium dioxide potentiostatic electrodeposition (cathodic current efficiency, current density, and product deposition rate) in electrolyte system M2WO4-M2W2O7-UO2WO4 (M = Li, Na, K, Cs) was studied in air. A conclusion was made that optimum electrodeposition and production of deposits not contaminated with supporting electrolyte decomposition products need higher cathodic polarization close to the solvent decomposition potentials and higher temperatures (up to 900°C). A melt based on K2WO4 was selected as the electrolyte providing satisfactory electrodeposition parameters of uranium dioxide with the composition close to stoichiometric.
Similar content being viewed by others
References
Afonichkin, V.K., Komarov, V.E., and Khrustova, L.G., Radiokhimiya, 2006, vol. 48, no. 2, p. 128 [Radiochemistry (Engl. Transl.), vol. 48, no. 2, p.].
Afonichkin, V.K., Komarov, V.E., and Vakarin, S.V., Elektrokhimiya, 1993, vol. 29, p. 1356 [Russ. J. Electrochem. (Engl. Transl.), vol. 29, p.].
Afonichkin, V.K., Komarov, V.E., and Khrustova, L.G., Elektrokhimiya, 2007, vol. 43, p. 921 [Russ. J. Electrochem. (Engl. Transl.), vol. 43, p.].
Afonichkin, V.K., Komarov, V.E., Leont’ev, V.N., and Nekrasova, N.P., Elektrokhimiya, 1996, vol. 32, p. 800 [Russ. J. Electrochem. (Engl. Transl.), vol. 32, p.].
Afonichkin, V.K., Komarov, V.E., Khrustova, L.G., Nekrasova, N.P., and Leont’ev, V.N., Tez. dokl. XI konf. po fiz. khimii i elektrokhimii rasplavlennykh i tverdykh elektrolitov (Abstracts of XI Conference on Physical Chemistry and Electrochemistry of Molten and Solid Electrolytes), Yekaterinburg: IVTE UrO RAN, 1998, vol. 1, p. 73.
Komarov, V.E. and Nekrasova, N.P., Elektrokhimiya, 1981, vol. 27, p. 1266.
Schreiber, H.D., Balazs, G.B., and Kosak, S.J., J. Am. Ceram. Soc., 1983, vol. 66, no. 5, p. 340.
Schreiber, H.D. and Balazs, G.B., Phys. and Chem. Glasses, 1982, vol. 23, no. 5, p. 139.
Schreiber, H.D., Balazs, G.B., and Jamison, P.L., Shaffer QA.P, Phys. Chem. Glasses, 1982, vol. 23, no. 5, p. 147.
Schreiber, H.D. and Balazs, G.B., J.Non-Cryst. Solids, 1982, vol. 49, no. 1, p. 189.
Afonichkin, V.K., Khrustova, L.G., and Semenovykh, V.V., Tez. dokl. XIII Ros. konf. po fiz. khimii i elektrokhimii rasplavlennykh i tverdykh elektrolitov (Abstracts of XIII Russian Conference on Physical Chemistry and Electrochemistry of Molten and Solid Electrolytes), Yekaterinburg: IVTE UrO RAN, 2004, vol. 1. p. 189.
Afonichkin, V.K., Khrustova, L.G., and Semenovykh, V.V., Tez. dokl. V seminara SO RAN — UrO RAN “Termodinamika i materialovedenie” (Abstracts of V Seminar of Siberian and Ural Divisions of the Russian Academy of Sciences on Thermodynamics and Materials Science, Novosibirsk: INKh SO RAN, 2005, p. 9.
Afonichkin, V.K. and Khrustova, L.G., Tez. dokl. Vserossiiskoi konf. “Khimiya tverdogo tela i funktsional’nye materialy” (Abstracts of All-Russian Conference on Solid State Chemistry of Functional Materials), Yekaterinburg: IKhTT UrO RAN, 2008, p. 21.
Afonichkin, V.K. and Khrustova, L.G., Tez. dokl. XIV Rossiiskoi konf. po fizicheskoi khimii i elektrokhimii rasplavlennykh i tverdykh elektrolitov (Abstracts of XIV Russian Conference on Physical Chemistry and Electrochemistry of Molten and Solid Electrolytes), Yekaterinburg: IVTE UrO RAN, 2007, vol. 1, p. 18.
Mokhosoev, M.V., Alekseev, F.P., and Lutsyk, V.I., Diagrammy Sostoyaniya Molibdatnykh i Vol’framatnykh Sistem (State Diagrams of Molybdate and Tungstate Systems), Novosibirsk: Nauka, 1978.
Nomure, Y., Kamegashira, N., and Naito, K., J. Ñrystal Growth, 1981, vol. 52, p. 279.
Afonichkin, V.K., Komarov, V.E., Khrustova, L.G., and Vakarin, S.V., Radiokhimiya, 2001, vol. 43, no. 3, p. 224 [Radiochemistry (Engl. Transl.), vol. 43, no. 3, p.].
Smirnov, M.V., Elektrodnye potentsialy v rasplavlennykh khloridakh (The Electrode Potentials in Molten Chlorides), Moscow: Nauka, 1973.
Zakhar’yash, S.M., Extended Abstract of Cand. Sci. (Chem.) Dissertation, Sverdlovsk: In-t elektrokhimii UNTs AN SSSR, 1982.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.K. Afonichkin, L.G. Khrustova, 2010, published in Elektrokhimiya, 2010, Vol. 46, No. 6, pp. 693–699.
Rights and permissions
About this article
Cite this article
Afonichkin, V.K., Khrustova, L.G. Effect of cationic composition of melts M2WO4-M2W2O7-UO2WO4 (M = Li, Na, K, Cs) and parameters of electrolysis to uranium dioxide electrodeposition characteristics. Russ J Electrochem 46, 652–658 (2010). https://doi.org/10.1134/S1023193510060091
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193510060091