Abstract
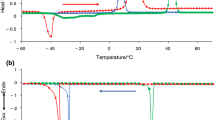
Effects of environment conditions (humidity and temperature) on the proton conductivity of aminobenzenesulfonic acids: 2-amino-(orthanilic) acid (I), 3-amino-(metanilic) acid (II), 4-amino-(sulfanilic) acid (III), their general formula NH2C6H4SO3H, and 3-amino-4-hydroxobenzenesulfonic acid (IV) [NH2(OH)C6H3SO3H), as well as (for sake of comparison) inorganic aminosulfonic acid [sulphamic acid (NH2SO3H)] (V) are studied. All above-listed compounds are zwitter-ions: they contain a fragment NH +3 SO −3 . The presence of this structural fragment affects the thermal stability of the compounds; according to the mass-spectrometry analysis data, the decomposition of the SO3-fragment begins at the following temperatures: (I) −339, (II) −370, (III) −320, (IV) −278, and (V) −220°C. It is shown that the increase of the environment relative humidity up to 95% results in the increase of the aminobenzenesulfonic acids proton conductivity from 10−9–10−8 to 10−5 S cm−1; sulphamic acid, to 10−4 S cm−1. At that, the amount of adsorbed water does not exceed 0.2 moles per 1 sulfo group in all cases. The conductance activation energy equals 0.2 eV at a relative humidity of 95%.
Similar content being viewed by others
References
Rusanov, A.L., Likhatchev, D., Kostoglodov, P.V., et al., Adv. Polym. Sci., 2005, vol. 179, p. 83.
Ukshe, E.A., Leonova, L.S., and Mikhailova, A.M., Elektrokhimiya, 1988, vol. 24, p. 110.
Filipenko, O.S., Chuev, I.I., Leonova, L.S., Shilov, G.V., and Aldoshin, S.M., Dokl. Akad. Nauk SSSR, 2001, no. 4, p. 501 [Dokl. (Engl. Transl.), no. 4, p. 27].
Bukun, N., Rodionov, V., and Mikhailova, A.M., Solid State Ionics, 1997, vol. 97, p. 257.
Pisareva, A.V., Shilov, G.V., Karelin, A.I., Pisarev, R.V., and Dobrovol’skii, Yu.A., Izv. Akad. Nauk, Ser. Khim., 2008, no. 2, p. 356.
Pisareva, A.V., Shilov, G.V., Karelin, A.I., and Dobrovol’skii, Yu.A., Zh. Fiz. Khim., 2008, vol. 82, no. 3 p. 433 [Russ. J. Phys. Chem. (Engl. Transl.), vol. 82, no. 3, p. 355].
Bukun, N.G., Ukshe, A.E., and Ukshe, E.A., Elektrokhimiya, 1993, vol. 29, p. 110 [Russ. J. Electrochem. (Engl. Transl.), vol. 29, p. 100].
Kanda, F.A. and King, A.J., J. Am. Chem. Soc., 1951, vol. 73, p. 2315.
Sass, R.L., Acta Crystallogr., 1960, vol. 13, p. 320.
Hall, S.R. and Maslen, E.N., Acta Crystallogr., 1967, vol. 22, no. 2, p. 216.
Hall, S.R. and Maslen, E.N., Acta Crystallogr., 1965, vol. 18, no. 3, p. 301.
Low, J.N. and Glidewell, C., Acta Crystallogr. Sect.C., 2002, vol. 58, p. 209.
Gunderman, B.J. and Squattrio, P.J., Acta Crystallogr. Sect.C., 1996, vol. 52, p. 940.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Pisareva, R.V. Pisarev, Yu.A. Dobrovol’skii, 2009, published in Elektrokhimiya, 2009, Vol. 45, No. 6, pp. 740–743.
Published by report at IX Conference “Fundamental Problems of Solid State Ionics”, Chernogolovka, 2008.
Rights and permissions
About this article
Cite this article
Pisareva, A.V., Pisarev, R.V. & Dobrovol’skii, Y.A. Effect of the air humidity on the proton conductivity of some aminobenzenesulfonic acids. Russ J Electrochem 45, 693–696 (2009). https://doi.org/10.1134/S1023193509060111
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193509060111