Abstract
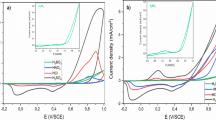
The preparation method of a self-supporting doped-polyaniline film electrode and its open-circuit potential (OCP) in NaClO4 and Na2SO4 solutions with different pH value as well as cathodic polarization behavior have been investigated for the purpose of discussing the corrosion electrochemical behavior of polyaniline (PANI) in the acid solution. X-ray photoelectron spectroscopy (XPS) reveals that the lower pH corresponds to higher doping level of H+ in the film and a more positive OCP of PANI film electrode. OCP of the PANI film reached 0.35 V vs. SCE in 1M H2SO4, which is more positive than that of most metals, suggests that PANI would act as cathode when it couples with these metals. The cathodic polarization experiments indicate that the dominating cathodic polarization process of PANI is reversible doping and dedoping reaction and the reduction of dissolve oxygen has very little contribution to it. The potentiostatic current-time curves exhibit a large transient current density at initial stage of polarization, which should be attributed to the charge stored in the film and a relative less steady state current density at the subsequent stage of polarization, which is provided by its doping/dedoping equilibrium activity. Such a current characteristic of PANI electrode might be the force of PANI to provide the passivation protection for some active-passive metals.
Similar content being viewed by others
References
DeBerry, D.W., J. Electrochem. Soc., 1985, vol. 132, p. 1022.
Cook, A., Gabriel, A., Siew, D., and Laycock, N., Current Applied Physics, 2004, vol. 4, p. 133.
Beck F., Haase, V., and Schroetz, M., AIP Conf. Proc., 1996, vol. 354, p. 115.
Wessling, B., Adv. Mater, 1994, vol. 6, p. 226.
Kinlen, P.J., Menon, V., and Ding, Y.W., J. Electrochem. Soc., 1999, vol. 146, p. 3690.
Malik, M.A., Galkowski, M.T., Bala, H., Grzybowska, B., and Kulesza, P.J., Electrochim. Acta, 1999, vol. 44, p. 2157.
Li P., Tan, T.C., and Lee, J.Y., Synth. Met., 1997, vol. 88, p. 237.
Fahlman, M., Jasty, S., and Epstein, A.J., Synth. Met., 1997, vol. 85, p. 1323.
Schauer, T., Joos, A., Dulog, L., and Eisenbach, C.D., Prog. Org. Coat., 1998, vol. 33, p. 20.
Talo, A., Passiniemi, R., Forsen, O., and Ylasaari, S., Synth. Met., 1997, vol. 85, p. 1333.
Ahmad, N. and MacDiarmid, A.G., Synth. Met., 1996, vol. 78, p. 103.
Pud, A.A., Shapoval, G.S., Kamarchik, P., Ogurtsov, N.A., Gromovaya V.F., Myronyuk, I.E., and Kontsur, Yu.V., Synth. Met., 1999, vol. 107, p. 111.
Lu, W.K., Elsenbaumer, R.L., and Wessling, B., Synth. Met., 1995, vol. 71, p. 2163.
Santiago, E.I., Pereira, E.C., and Bulhoes, L.O.S., Synth. Met., 1998, vol. 98, p. 87.
Fraoua, K., Delamar, M., and Andrieux, C.P., J. Electroanal. Chem., 1996, vol. 418, p. 109.
Kang, E.T., Neoh, K.G., and Tan, K.L., Synth. Met., 1995, vol. 68, p. 141.
Ping, Z., Neugebauer, H., and Neckel, A., Synth. Met., 1995, vol. 69, p. 161.
Luthra, V., Singh, R., Gupta, S.K., and Mansingh, A., Current Applied Physics, 2003, vol. 3, p. 219.
Lubert, K.-H. and Dunsch, L., Electrochim. Acta, 1998, vol. 43, p. 813.
Neudeck, A., Petr, A., and Dunsch, L., Synth. Met., 1999, vol. 107, p. 143.
Ping, Z., Nauer, G.E., Neugebauer, H., Theiner, J., and Neckel, A., Electrochim. Acta, 1997, vol. 42, p. 1693.
Ray, A., Asturias, G.E., Kershner, D.L., MacDiarimid, A.G., and Epstein, A.J., Synth. Met., 1989, vol. 29, p. E141.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2008, Vol. 44, No. 10, pp. 1205–1212.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Zhu, H., Hu, J., Zhong, L. et al. Cathodic polarization behavior of self-supporting polyaniline film electrode. Russ J Electrochem 44, 1120–1126 (2008). https://doi.org/10.1134/S1023193508100066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193508100066