Abstract
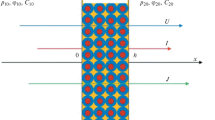
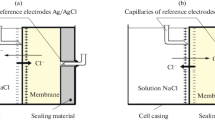
Structural and mathematical models are proposed for describing electrolyte transport through heterogeneous anion-exchange membranes under conditions of pressure-dependent electrodiffusion. The idea that mesopores and macropores are present in the membrane provides the basis for the structural model. The Nernst-Planck equations with a convective term are used to describe ion transport in the solution filling the pores. Results of the solution to the mathematical problem and the experimental investigations demonstrate the possibility of decreasing the transport numbers of sodium ions through an anion-exchange membrane by applying a pressure gradient in the same direction as the electrolyte diffusion flux in the membrane.
Similar content being viewed by others
References
Glueckauf, E., Proc. R. Soc. London, Ser. A, 1962, vol. 268, p. 350.
Gnusin, N.P., Grebenyuk, V.D., and Pevnitskaya, M.V., Elektrokhimiya ionitov (The Electrochemistry of Ion-Exchange Resins), Novosibirsk: Nauka, 1972.
Gnusin, N.P., Zabolotskii, V.I., Nikonenko, V.V., and Meshechkov, A.I., Zh. Fiz. Khim., 1980, vol. 54, p. 1518.
Zabolotsky, V., and Nikonenko, V., J. Membr. Sci., 1993, vol. 79, p. 181.
Vuoristo, M., Kontturi, K., Manzanares, Kh.A., Mafe, S., Elektrokhimiya, 1996, vol. 32, p. 192.
Koter, S., J. Membr. Sci., 2002, vol. 206, p. 201.
Quenneville, E., and Buschmann, M., J. Membr. Sci., 2005, vol. 265, p. 60.
Helfferich, F., Ion Exchange, New York: McGraw-Hill, 1962.
Lakshminarayanaiah, N., Transport Phenomena in Membranes, New York: Academic, 1969.
Schmid, G., J. Membr. Sci., 1998, vol. 150, p. 159.
Mafe, S., Manzanares, J., and Pellicer, J., J. Membr. Sci., 1990, vol. 51, p. 161.
Pevnitskaya, M.V., Starikovskii, L.G., Usov, V.Yu., and Borodikhina, L.I., Zh. Prikl. Khim., 1981, vol. 54, p. 2077.
Grebenyuk, V.D., and Ponomarev, M.I., Elektromembrannoe razdelenie smesei (Electromembrane Separation of Mixtures), Kiev: Naukova Dumka, 1992.
Berezina, N.P., Vol’fkovich, Yu.M., Kononenko, N.A., and Blinov, I.A., Elektrokhimiya, 1987, vol. 23, p. 912.
Pis’menskii, V.F., Zabolotskii, V.I., and Gnusin, N.P., Abstracts of Papers, Vsesoyuznoe soveshchanie “Primenenie elektrodializa v membranno-sorbstionnoi tekhnologii ochistki i razdeleniya veshchestv” (All-Union Conference “The Application of Electrodialysis in Membrane-Sorption Technology of Purification and Separation of Substances”) Cherkassy, 1984, p. 40.
Aristov, I.V., Bobreshova, O.V., and Balavadze, E.M., Elektrokhimiya, 1996, vol. 32, p. 1112.
Lazarev, S.I., and Vyazov, S.A., Khim. Khim. Tekhnol., 2005, vol. 48, No. 3, p. 91.
Shel’deshov, N.V., Chaika, V.V., and Zabolotskii, V.I., Sorbts. khromatogr. protsessy, 2007, vol. 7, No. 1, p. 5.
Zhang, G., Grabowski, A., Strathmann, H., and Eigenberger, G., Abstracts of Papers, International Congress on Membranes and Membranes Processes 2005 (ICOM 2005), August 21–26, 2005, Seoul, South Korea, 2005, PW-311 (Abstract no. 695), p. 1407.
Pivovarov, N.Ya., Geterogennye ionoobmennye membrany v elektrodializnykh protsessakh (Heterogeneous Ion-Exchange Membranes in Electrodialysis Processes), Vladivostok: Dal’nauka, 2001.
Chaika, V.V., Shel’deshov, N.V., and Zabolotskii, V.I., Abstracts of Papers, Rossiiskaya konferenstiya-shkola s mezhdunarodnym uchastieum (Russian Conference-School with International Participation), May 22–25, 2007, Krasnodar, 2007, p. 182.
Mazin, V.M., Sobalev, V.D., Vol’fkovich, Yu.M., and Churaev, N.V., Elektrokhimiya, 1984, vol. 28, p. 953.
Gnusin, N.P., Berezina, N.P., and Demina, O.A., Zh. Prikl. Khim., 1986, vol. 57, p. 679.
Damaskin, B.B., and Petrii, O.A., Vvedenie v elektrokhimichesuyu kinetiku (Introduction to Electrochemical Kinetics), Moscow: Vysshaway shkola, 1983.
Robinson, R.A., and Stokes, R.H., Electrolyte Solutions, 2nd ed. rev., London: Butterworths, 1965.
Bell, R.P., The Proton in Chemistry, 2nd ed., Ithaca, N.Y.: Cornell Univ. Press, 1973.
Mauro, A., Biohys. J., 1962, vol. 2, p. 179.
Fridrikhsberg, D.A., Kurs kolloidnoi khimii (Course of Colloid Chemistry), Leningrad: Khimiya, 1984.
Zabolotskii, V.I., and Nikonenko, V.V., Perenos ionov v membrannakh (Ion Transport in Membranes), Moscow: Nauka, 1996.
Landau, L.D., and Lifshitz, E.M., Gidronamika, vol. 6 of Teoreticheskaya fizika, Moscow: Nauka, 1986 [Fluid Mechanics (Engl. Transl.), 2nd ed. rev., vol. 6 of Course of Theoretical Physics, Oxford: Pergamon, 1987].
Shaposhnik, V.A., Sorosov. Obraz. Zh., 1999, No. 9, p. 27.
Fary, A.D., Jr., The Diffusional Properties of Sodium Hydroxide, Ph.D. Dissertation, Institute of Paper Science and Technology, Appleton, Wis., 1966.
Spravochnik khimika (Chemist’s Handbook), Nikol’skii, B.P., Ed., Moscow: Khimiya, 1964, vol. 3.
Krasovskii, G.I., and Filaretov, G.F., Planirovanie eksperimenta (Planning the Experiment), Minsk: Beloruss. Gos. Univ., 1982.
Vol’fkovich, Yu.M., Elektrokhimiya, 1984, vol. 20, p. 665.
Grigorov, O.N., Koz’mina, Z.P., Markovich, A.V., and Fridrikhsberg, D.A., Elektrokineticheskie svoistva kapillyarnykh sistem (Electrokinetic Properties of Capillary Systems), Rebinder, P.A., Ed., Moscow: Akad. Nauk SSSR, 1956.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.V. Shel’deshov, V.V. Chaika, V.I. Zabolotskii, 2008, published in Elektrokhimiya, 2008, Vol. 44, No. 9, pp. 1116–1126.
Rights and permissions
About this article
Cite this article
Shel’deshov, N.V., Chaika, V.V. & Zabolotskii, V.I. Structural and mathematical models for pressure-dependent electrodiffusion of an electrolyte through heterogeneous ion-exchange membranes: Pressure-dependent electrodiffusion of NaOH through the MA-41 anion-exchange membrane. Russ J Electrochem 44, 1036–1046 (2008). https://doi.org/10.1134/S1023193508090085
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193508090085