Abstract
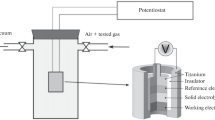
Effect the morphology of the surface of the working electrode (PbS) exerts on the sensitivity of a low-temperature potentiometric hydrogen sulfide sensor is studied. The sensor, which is based on electrochemical cell Na x WO3/NASICON/PbS, may be used for fast selective detection of hydrogen sulfide in air in natural conditions. It is demonstrated that the sensors with PbS that are deposited out of solution have a faster response than the pressed-to ones. The dependence of EMF on the hydrogen sulfide concentration for the former is linear in semilogarithmic coordinates. Thus difference is explained by the microstructure of the lead sulfide layer. It is shown that the lead sulfide interaction with hydrogen sulfide involves a reversible partial reduction of sulfur and lead at the surface. The species that form in so doing contain sulfur atoms in lower oxidation degrees (poly-and oligo sulfides, sulfite). A mechanism of the sensor operation is proposed on the basis of data yielded by experiment and quantum-chemical simulation. The mechanism includes reversible transport of hydrogen from sulfur atoms to oxygen atoms.
Similar content being viewed by others
References
Sutherland, F.M., Etsell, T.H., and Eastman, C.D., Solid State Ionics, 1992, vol. 53–56, p. 68.
Yan, Y.T., Miura, N., and Yamazoe, N., Chem. Lett., 1994, vol. 9, p. 1753.
Miura, N., Yan, Y.T., Lu, G.Y., and Yamazoe, N., Sens. Actuators, B 1996, vol. 34, p. 367.
Vandecruys, F., Stephen, R., De Schutter, F., and Vangrunderbeek, J., Sens. Actuators, B 1997, vol. 43, p. 230.
Vandecruys, F., Brauns, E., Engelen, W., de Schutter, F., and Vangrunderbeek, J., Solid State Ionics, 1998, vol. 112, p. 95.
Vangrunderbeek, J., Vandecruys, F., and Kumar, R.V., Sens. Actuators, B 1999, vol. 56, p. 129.
Yu, C.B., Wang, Y.J., Hua, K.F., Xing, W., and Lu, T.H., Sens. Actuators, B 2002, vol. 86, p. 259.
Wang, Y.R., Yan, H.Q., and Wang, E., Sens. Actuators, B 2002, vol. 87, p. 115.
Kirchnerova, J., Bale, C.W., and Skeaff, J.M., Solid State Ionics, 1996, vol. 91, p. 257.
Nagashima, K. and Shigetaka, S., Bunseki Kagaku, 1983, vol. 32, p. 219.
Leonova, L., Dobrovolsky, Yu., Ukshe, E., Tkacheva, N., and Gabrel’yan, A., Metrologiya, 1991, vol. 6, p. 45.
Dobrovolsky, Yu., Leonova, L., and Vakulenko, A., Solid State Ionics, 1996, vol. 86–88, p. 1017.
Dobrovolsky, Yu.A., Leonova, L.S., and Vakulenko, A.M., Elektrokhimiya, 1996, vol. 32, p. 438.
Bukun, N., Dobrovolsky, Y., Levchenko, A., Leonova, L., and Osadchii, E., J. Solid State Electrochem., 2003, vol. 7, p. 122.
Bukun, N.G., Domashnev, I.A., Moskvina, E.I., and Ukshe, E.A., Izv. Akad. Nauk SSSR, Neorg. Mater., 1988, vol. 24, p. 443.
Handbuch der Praeparativen Anorganishen Chemie, Brauer, G., Ed., Stuttgart: Ferdinand Enke, 1978, vol. 2.
Laajalehto, K., Kartio, I., and Suoninen, E., Int. J. Miner. Process., 1997, vol. 51, p. 163.
Nowak, P. and Laajalehto, K., App. Surf. Sci., 2000, vol. 157, p. 101.
Gaussian 98, Pittsburg (PA): Gaussian, Inc., 1998, revision A.
Ravindra, N.M. and Srivastava, V.K., Phys. Status Solidi A, 1980, vol. 58, p. 311.
Zyubina, T.S., Neudachina, V.S., Yashina, L.V., and Shtanov, V.I., Surf. Sci., 2005, vol. 574, p. 52.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.V. Levchenko, Yu.A. Dobrovolsky, N.G. Bukun, L.S. Leonova, T.S. Zyubina, V.S. Neudachina, L.V. Yashina, A.B. Tarasov, T.B. Shatalova, V.I. Shtanov, 2007, published in Elektrokhimiya, 2007, Vol. 43, No. 5, pp. 584–592.
Based on the paper delivered at the 8th Meeting “Fundamental Problems of Solid-State Ionics”, Chernogolovka (Russia), 2006.
Rights and permissions
About this article
Cite this article
Levchenko, A.V., Dobrovolsky, Y.A., Bukun, N.G. et al. Chemical and electrochemical processes in low-temperature superionic hydrogen sulfide sensors. Russ J Electrochem 43, 552–560 (2007). https://doi.org/10.1134/S1023193507050084
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1023193507050084