Abstract
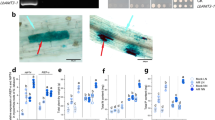
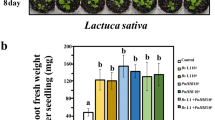
Previous studies have proved that arbuscular mycorrhizal fungi infection and phosphate transporters are particularly important for phosphorus uptake by plants. Early laboratory studies found that LbPT7 played a role in Pi uptake and it was affected by arbuscular mycorrhizal fungi. In order to verify the phosphorus uptake function of LbPT7 gene and the effect of interaction with arbuscular mycorrhiza on plants, we constructed LbPT7 overexpression vector with Nicotiana tabacum and obtained T1 generation overexpression positive plants as test material. Under the condition of pot experiment, we designed three factors: Arbuscular Mycorrhizal Fungi treatment, genotype treatment, phosphorus concentration treatment. After 4 weeks of treatment, we measured the biomass and phosphorus content of tobacco, and determined the colonization. The results showed that overexpression of LbPT7 can promote plant phosphorus uptake and inoculation of AM fungi further promote plant phosphorus uptake in phosphorus deficiency condition, but this effect of them can’t superpose in phosphorus adequate conditions. Overexpression of LbPT7 promoted the absorption of phosphorus, but impeded the utilization of phosphorus of N. tabacum in phosphorus deficiency condition. Overexpression of LbPT7 had little effect on the colonization of AM fungi in N. tabacum.


Similar content being viewed by others
REFERENCES
Li, Z.X., Ni, Z.F., Peng, H.R., et al., Molecular mapping of QTLs for root response to phosphorus deficiency at seedling stage in wheat (Triticum aestivum L.), Prog. Nat. Sci., 2007, vol. 17, no. 10, pp. 177—184.
Wang, Y., Chen, Y.F., and Wu, W.H., Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol., 2021, vol. 63, no. 1, pp. 34—52.
Ch’ng, H.Y., Ahmed, O.H., and Ab Majid, N.M., Improving phosphorus availability in an acid soil using organic amendments produced from agroindustrial wastes, Sci. World J., 2014, vol. 20. https://doi.org/10.1155/2014/506356
Richardson, A.E., Hocking, P.J., Simpson, R.J., and George, T.S., Plant mechanisms to optimise access to soil phosphorus, Crop Pasture Sci., 2009, vol. 60, no. 2, pp. 124—143.
Grant, C., Bittman, S., Montreal, M., et al., Soil and fertilizer phosphorus: effects on plant P supply and mycorrhizal development, Can. J. Plant Sci., 2005, vol. 85, no. 1, pp. 3—14.
Nagahashi, G. and Douds, D., Partial separation of root exudates components and their effects upon the growth of germinated spores of AM fungi, Mycol. Res., 2000, vol. 104, pp. 1453—1464.
Smith, S.E., Smith, F.A., and Jakobsen, I., Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses, Plant Physiol., 2003, vol. 133, no. 1, pp. 16—20.
Smith, S.E. and Read, D.J., Mycorrhizal Symbiosis, Amsterdam: Elsevier, 2008.
Cao, M.A., Liu, R.C., Xiao, Z.Y., et al., Symbiotic fungi alter the acquisition of phosphorus in Camellia oleifera through regulating root architecture, plant phosphate transporter gene expressions and soil phosphatase activities, J. Fungi, 2022, vol. 8, no. 8.
Grant, C., Bittman, S., Montreal, M., et al., Soil and fertilizer phosphorus: effects on plant P supply and mycorrhizal development, Can. J. Plant Sci., 2005, vol. 85, no. 1, pp. 3—14.
De-los-Santos, R.T., Rosales, N.M., Ocampo, J.A., and Garcia-Garrido, J.M., Ethylene alleviates the suppressive effect of phosphate on arbuscular mycorrhiza formation, J. Plant Growth Regul., 2016, vol. 35, no. 3, pp. 611—617.
Schachtman, D.P., Reid, R.J., and Ayling, S.M., Phosphorus uptake by plants from soil to cell, Plant Physiol., 1998, vol. 116, no. 2, pp. 447—453.
Wei, X.S., Fu, Y., Yu, R.J., et al., Comprehensive sequence and expression profile analysis of the phosphate transporter gene family in soybean, Sci. Rep., 2022, vol. 12, no. 1.
Koyama, T., Ono, T., Shimizu, M., et al., Promoter of Arabidopsis thaliana phosphate transporter gene drives root-specific expression of transgene in rice, J. Biosci. Bioeng., 2005, vol. 99, no. 1, pp. 38—42.
Xiao, K., Liu, J., Dewbre, G., et al., Isolation and characterization of root-specific phosphate transporter promoters from Medicago truncatula, Plant Biol., 2006, vol. 8, no. 4, pp. 439—449.
Chang, M.X., Gu, M., Xia, Y.W., et al., OsPHT1; 3 mediates uptake, translocation, and remobilization of phosphate under extremely low phosphate regimes, Plant Physiol., 2019, vol. 179, no. 2, pp. 656—670.
Xie, X.A., Huang, W., Liu, F.C., et al., Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis, New Phytol., 2013, vol. 198, no. 3, pp. 836—852.
Grønlund, M., Albrechtsen, M., Johansen, I.E., et al., The interplay between P uptake pathways in mycorrhizal peas: a combined physiological and gene-silencing approach, Physiol. Plant., 2013, vol. 149, no. 2, pp. 234—248.
Madrid-Delgado, G., Orozco-Miranda, M., Cruz-Osorio, M., et al., Pathways of phosphorus absorption and early signaling between the mycorrhizal fungi and plants, Phyton—Int. J. Exp. Bot., 2021, vol. 90, no. 5, pp. 1321—1338.
Hu, W.T., Zhang, H.Q., Zhang, X.Y., et al., Characterization of six PHT1 members in Lycium barbarum and their response to arbuscular mycorrhiza and water stress, Tree Physiol., 2017, vol. 37, no. 3, pp. 351—366.
Cheng, K., Wei, M., Jin, X.X., et al., LbAMT3-1, an ammonium transporter induced by arbuscular mycorrhizal in Lycium barbarum, confers tobacco with higher mycorrhizal levels and nutrient uptake, Plant Cell Rep., 2022, vol. 41, no. 6, pp. 1477—1480.
Jyothishwaran, G., Kotresha, D., Selvaraj, T., et al., A modified freeze-thaw method for efficient transformation of Agrobacterium tumefaciens, Curr. Sci., 2007, vol. 93, no. 6, pp. 770—772.
Horsch, R.B., Fry, J.E., Hoffmann, N.L., et al., A simple and general method for transferring genes into plants, Science, 1985, vol. 227, pp. 1229—1231.
Koske, R.E. and Gemma, J.N., A modified procedure for staining roots detect VA mycorrhizas. Mycol. Res., 1989, vol. 92, no. 4, pp. 486—488.
McGonigle, T.P., Miller, M.H., Evans, D.G., et al., A new method which gives an objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi, New Phytol., 1990, vol. 115, no. 3, pp. 495—501.
Bao, S.D., Agrochemical Analysis of Soil, Beijing: China Agricultural Press, 2000.
Wang, P., Wu, T.Y., Wen, S.H., et al., Effect of arbuscular mycorrhizal fungi on rhizosphere organic acid content and microbial activity of trifoliate orange under different low P conditions, Arch. Agron. Soil Sci., 2019, vol. 65, no. 14, pp. 2029—2042.
Khan, Y., Shah, S., and Tian, H., The roles of arbuscular mycorrhizal fungi in influencing plant nutrients, photosynthesis, and metabolites of cereal crops—a review, Agronomy (Basel), 2022, vol. 12.
Akiyama, K., Matsuoka, H., and Hayashi, H., Isolation and identification of a phosphate deficiency-induced C-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots, Mol. Plant—Microbe Interact., 2002, vol. 15, no. 4, pp. 334—340.
Das, D., Paries, M., Hobecker, K., et al., Phosphate starvation response transcription factors enable arbuscular mycorrhiza symbiosis, Nat. Commun., 2022, vol. 13, no. 1.
Rausch, C. and Bucher, M., Molecular mechanisms of phosphate transport in plants, Planta, 2002, vol. 216, pp. 23—37.
Liao, D.H., Sun, C., Liang, H.Y., et al., SlSPX1-SlPHR complexes mediate the suppression of arbuscular mycorrhizal symbiosis by phosphate repletion in tomato, Plant Cell, 2022, vol. 34, no. 10, pp. 4045—4065.
Nagy, R., Karandashov, V., Chague, W., et al., The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species, Plant J., 2005, vol. 42, no. 2, pp. 236—250.
Tan, Z.J., Hu, Y.L., and Lin, Z.P., Expression of NtPT5 is correlated with the degree of colonization in tobacco roots inoculated with Glomus etunicatum, Plant Mol. Biol. Rep., 2012, vol. 30, no. 4, pp. 885—893.
Chen, A.Q., Hu, J., Sun, S.B., and Xu, G.H., Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species, New Phytol., 2007, vol. 173, no. 4, pp. 817—831.
Smith, S.E., Jakobsen, I., Gronlund, M., and Smith, F.A., Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition, Plant Physiol., 2011, vol. 156, no. 3, pp. 1050—1057.
Hinsinger, P., Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review, Plant Soil, 2001, vol. 237, no. 2, pp. 173—195.
Chrysargyris, A., Panayiotou, C., and Tzortzakis, N., Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.), Ind. Crops Prod., 2016, vol. 83, pp. 577—586.
Elser, J.J., Fagan, W.F., Kerkhof, A.J., et al., Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change, New Phytol., 2010, vol. 186, no. 3, pp. 593—608.
Yang, Z.J., Wu, X.H., Chen, L.H., et al., Fertilization regulates accumulation and allocation of biomass and nutrients in Phoebe bournei seedlings, Agriculture (Basel), 2022, vol. 11, no. 12.
Yang, Q., Li, Q., Zhang, J.B., et al., Phosphorus addition increases aboveground biomass but does not change N:P stoichiometry of Chinese fir (Cunninghamia lanceolata) seedlings under nitrogen deposition, Pol. J. Environ. Stud., 2021, vol. 30, no. 2, pp. 1421—1431.
Tian, C.J., He, X.Y., Zhong, Y., and Chen, J.K., Effect of inoculation with ecto- and arbuscular mycorrhizae and Rhizobium on the growth and nitrogen fixation by black locust, Robinia pseudoacacia, New For., 2003, vol. 25, no. 2, pp. 125—131.
Shin, H., Shin, H.S., Dewbre, G.R., and Harrison, M.J., Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments, Plant J., 2004, vol. 39, no. 4, pp. 629—642.
Funding
This research was funded by the National Natural Science Foundation of China (42277027, 31700530), the State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources (SKLCUSA-b202007).
Author information
Authors and Affiliations
Contributions
Yanpeng Li: Conceptualization, Investigation Data curation, Methodology, Software, Visualization, Writing— original draft, Writing—review and editing. Xia Han: Conceptualization, Investigation, Methodology, Software. Yuhao Zhou: Investigation, Software, Visualization. Ming Tang: Supervision, Funding acquisition, Project administration. Haoqiang Zhang: Conceptualization, Supervision, Funding acquisition, Project administration, Writing— review and editing.
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Zhou, Y., Han, X. et al. Overexpression of LbPT7 Promotes Phosphorus Uptake by Plants and Affects Phosphorus Uptake by Arbuscular Mycorrhizas under High Phosphorus Condition. Russ J Genet 60, 588–594 (2024). https://doi.org/10.1134/S1022795424700108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795424700108