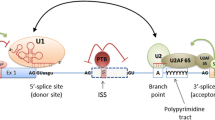
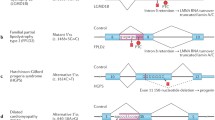
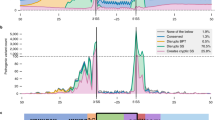
Abstract
Despite the development of exome and whole genome sequencing technologies and their routine use in the diagnosis of hereditary diseases, the efficiency of detection of pathogenic genetic variants for methods based on DNA analysis is less than 50%. One of the main reasons may be the inefficiency of these approaches in the search for genetic variants responsible for impaired pre-mRNA splicing. This review discusses the results of work on the search for splicing abnormalities in hereditary orphan diseases using RNA sequencing and the possibility of clinical application of this method.
Similar content being viewed by others
REFERENCES
https://www.orpha.net/.
Nguengang Wakap, S., Lambert, D.M., Olry, A., et al., Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database, Eur. J. Hum. Genet., 2020, vol. 28, no. 2, pp. 165—173. https://doi.org/10.1038/s41431-019-0508-0
Online Mendelian Inheritance in Man. https://www.omim.org/.
Choi, M., Scholl, U.I., Ji, W., et al., Genetic diagnosis by whole exome capture and massively parallel DNA sequencing, Proc. Natl. Acad. Sci. U.S.A., 2009, vol. 106, no. 45, pp. 19096—19101. https://doi.org/10.1073/pnas.0910672106
Valencia, C.A., Husami, A., Holle, J., et al., Clinical impact and cost-effectiveness of whole exome sequencing as a diagnostic tool: a pediatric center’s experience, Front. Pediatr., 2015, vol. 3. https://doi.org/10.3389/fped.2015.00067
Tan, T.Y., Dillon, O.J., Stark, Z., et al., Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions, JAMA Pediatr., 2017, vol. 171, no. 9, p. 855. https://doi.org/10.1001/jamapediatrics.2017.1755
Retterer, K., Juusola, J., Cho, M.T., et al., Clinical application of whole-exome sequencing across clinical indications, Genet. Med., 2016, vol. 18, no. 7, pp. 696—704. https://doi.org/10.1038/gim.2015.148
Lionel, A.C., Costain, G., Monfared, N., et al., Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test, Genet. Med., 2018, vol. 20, no. 4, pp. 435—443. https://doi.org/10.1038/gim.2017.119
Schwarze, K., Buchanan, J., Taylor, J.C., et al., Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature, Genet. Med., 2018, vol. 20, no. 10, pp. 1122—1130. https://doi.org/10.1038/gim.2017.247
Robertson, A.J., Tan, N.B., Spurdle, A.B., et al., Reanalysis of genomic data: an overview of the mechanisms and complexities of clinical adoption, Genet. Med., 2022, vol. 24, no. 4, pp. 798—810. https://doi.org/10.1016/j.gim.2021.12.011
Liu, P., Meng, L., Normand, E.A., et al., Reanalysis of clinical exome sequencing data, N. Engl. J. Med., 2019, vol. 380, no. 25, pp. 2478—2480. https://doi.org/10.1056/NEJMc1812033
Tan, N.B., Stapleton, R., Stark, Z., et al., Evaluating systematic reanalysis of clinical genomic data in rare disease from single center experience and literature review, Mol. Genet. Genomic Med., 2020, vol. 8, no. 11, pp. 1—19. https://doi.org/10.1002/mgg3.1508
Stenson, P.D., Mort, M., Ball, E.V., et al., The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies, Hum. Genet., 2017, vol. 136, no. 6, pp. 665—677. https://doi.org/10.1007/s00439-017-1779-6
López-Bigas, N., Audit, B., Ouzounis, C., et al., Are splicing mutations the most frequent cause of hereditary disease?, FEBS Lett., 2005, vol. 579, no. 9, pp. 1900—1903. https://doi.org/10.1016/j.febslet.2005.02.047
Jiang, W. and Chen, L., Alternative splicing: human disease and quantitative analysis from high-throughput sequencing, Comput. Struct. Biotechnol. J., 2021, vol. 19, pp. 183—195. https://doi.org/10.1016/j.csbj.2020.12.009
Kalsotra, A. and Cooper, T.A., Functional consequences of developmentally regulated alternative splicing, Nat. Rev. Genet., 2011, vol. 12, no. 10, pp. 715—729. https://doi.org/10.1038/nrg3052
Marco-Puche, G., Lois, S., Benítez, J., et al., RNA-seq perspectives to improve clinical diagnosis, Front. Genet., 2019, vol. 10. https://doi.org/10.3389/fgene.2019.01152
Scotti, M.M. and Swanson, M.S., RNA mis-splicing in disease, Nat. Rev. Genet., 2016, vol. 17, no. 1, pp. 19—32. https://doi.org/10.1038/nrg.2015.3
Wu, Z.-H., Tang, Y., and Zhou, Y., Alternative splicing events implicated in carcinogenesis and prognosis of thyroid gland cancer, Sci. Rep., 2021, vol. 11, no. 1, p. 4841. https://doi.org/10.1038/s41598-021-84403-6
Marin, J.J.G., Reviejo, M., Soto, M., et al., Impact of alternative splicing variants on liver cancer biology, Cancers (Basel), 2022, vol. 14, no. 1, p. 18. https://doi.org/10.3390/cancers14010018
Kim, B.-H., Woo, T.-G., Kang, S.-M., et al., Splicing variants, protein—protein interactions, and drug targeting in Hutchinson—Gilford progeria syndrome and small cell lung cancer, Genes (Basel), 2022, vol. 13, no. 2, p. 165. https://doi.org/10.3390/genes13020165
Wachs, A.S. and Bohne, J., Two sides of the same medal: noncoding mutations reveal new pathological mechanisms and insights into the regulation of gene expression, WIREs RNA, 2021, vol. 12, no. 1, pp. 1—21. https://doi.org/10.1002/wrna.1616
Anna, A. and Monika, G., Splicing mutations in human genetic disorders: examples, detection, and confirmation, J. Appl. Genet., 2018, vol. 59, no. 3, pp. 253—268. https://doi.org/10.1007/s13353-018-0444-7
Habara, Y., Takeshima, Y., Awano, H., et al., In vitro splicing analysis showed that availability of a cryptic splice site is not a determinant for alternative splicing patterns caused by +1G>A mutations in introns of the dystrophin gene, J. Med. Genet., 2009, vol. 46, no. 8, pp. 542—547. https://doi.org/10.1136/jmg.2008.061259
Sanz, D.J., Hollywood, J.A., Scallan, M.F., et al., Cas9/gRNA targeted excision of cystic fibrosis-causing deep-intronic splicing mutations restores normal splicing of CFTR mRNA, PLoS One, 2017, vol. 12, no. 9, p. e0184009. https://doi.org/10.1371/journal.pone.0184009
Symoens, S., Malfait, F., Vlummens, P., et al., A novel splice variant in the n-propeptide of COL5A1 causes an eds phenotype with severe kyphoscoliosis and eye involvement, PLoS One, 2011, vol. 6, no. 5. e20121. https://doi.org/10.1371/journal.pone.0020121
Weisschuh, N., Buena-Atienza, E., and Wissinger, B., Splicing mutations in inherited retinal diseases, Prog. Retin. Eye Res., 2021, vol. 80, p. 100874. https://doi.org/10.1016/j.preteyeres.2020.100874
Chen, M. and Manley, J.L., Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches, Nat. Rev. Mol. Cell Biol., 2009, vol. 10, no. 11, pp. 741—754. https://doi.org/10.1038/nrm2777
Xiong, H.Y., Alipanahi, B., Lee, L.J., et al., The human splicing code reveals new insights into the genetic determinants of disease, Science, 2015, vol. 347, no. 6218. https://doi.org/10.1126/science.1254806
Cummings, B.B., Marshall, J.L., Tukiainen, T., et al., Improving genetic diagnosis in Mendelian disease with transcriptome sequencing Genotype—Tissue Expression Consortium, Sci. Transl. Med., 2017, vol. 9, no. 386. https://doi.org/10.1126/scitranslmed.aal5209
Gonorazky, H.D., Naumenko, S., Ramani, A.K., et al., Expanding the boundaries of RNA sequencing as a diagnostic tool for rare Mendelian disease, Am. J. Hum. Genet., 2019, vol. 104, no. 3, pp. 466—483. https://doi.org/10.1016/j.ajhg.2019.01.012
Murdock, D.R., Dai, H., Burrage, L.C., et al., Transcriptome-directed analysis for Mendelian disease diagnosis overcomes limitations of conventional genomic testing, J. Clin. Invest., 2021, vol. 131, no. 1. https://doi.org/10.1172/jci141500
Maddirevula, S., Kuwahara, H., Ewida, N., et al., Analysis of transcript-deleterious variants in Mendelian disorders: implications for RNA-based diagnostics, Genome Biol., 2020, vol. 21, no. 1, p. 145. https://doi.org/10.1186/s13059-020-02053-9
Lee, H., Huang, A.Y., Wang, L., et al., Diagnostic utility of transcriptome sequencing for rare Mendelian diseases, Genet. Med., 2020, vol. 22, no. 3, pp. 490—499. https://doi.org/10.1038/s41436-019-0672-1
Wai, H.A., Lord, J., Lyon, M., et al., Blood RNA analysis can increase clinical diagnostic rate and resolve variants of uncertain significance, Genet. Med., 2020, vol. 22, no. 6, pp. 1005—1014. https://doi.org/10.1038/s41436-020-0766-9
Frésard, L., Smail, C., Ferraro, N.M., et al., Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts, Nat. Med., 2019, vol. 25, no. 6, pp. 911—919. https://doi.org/10.1038/s41591-019-0457-8
Jaganathan, K., Kyriazopoulou Panagiotopoulou, S., McRae, J.F., et al., Predicting splicing from primary sequence with deep learning, Cell, 2019, vol. 176, no. 3, pp. 535—548. e24. https://doi.org/10.1016/j.cell.2018.12.015
Cooper, T.A., Use of minigene systems to dissect alternative splicing elements, Methods, 2005, vol. 37, no. 4, pp. 331—340. https://doi.org/10.1016/j.ymeth.2005.07.015
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Federal Scientific and Technical Program for the Development of Genetic Technologies for 2019–2027, agreement no. 075-15-2021-1061, RF 193021X0029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Skryabin, N.A., Zhigalina, D.I. & Stepanov, V.A. The Role of Splicing in the Pathogenesis of Monogenic Diseases. Russ J Genet 58, 1208–1215 (2022). https://doi.org/10.1134/S1022795422100088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422100088