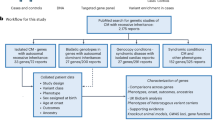
Abstract
The phenomenon of comorbidity between monogenic and multifactorial diseases suggests the involvement of a certain common number of genes and biological pathways in the formation of the predisposition to diseases, known as the Mendelian code, which links each multifactorial disease with a unique set of Mendelian loci. Within the omnigenic model of multifactorial diseases, genes of Mendelian diseases can be represented by core genes that function in the cells of the target organs of the pathology and participate in their pathogenesis. Mendelian diseases can be used as a starting point for prioritizing loci/genes related to complex traits and diseases. This approach was applied in this review by the example of prioritizing genes in loci associated with hypertrophic and dilated cardiomyopathies as a result of genome-wide association studies. The functional characteristics of the Mendelian disease genes in the genetic structure of the susceptibility to multifactorial diseases will provide new knowledge about core and peripheral genes and their areas of competence. It is important to analyze the Mendelian code of multifactorial diseases using the multiomic approach, which will allow one to identify driver genes and biological pathways associated with the development of diseases.

Similar content being viewed by others
REFERENCES
Timpson, N.J., Greenwood, C.M.T., Soranzo, N., et al., Genetic architecture: the shape of the genetic contribution to human traits and disease, Nat. Rev. Genet., 2018, vol. 19, pp. 110—124. https://doi.org/10.1038/nrg.2017.101
Dzau, V. and Braunwald, E., Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement, Am. Heart J., 1991, vol. 121, pp. 1244—1263. https://doi.org/10.1016/0002-8703(91)90694-d
Puzyrev, V.P., Makeeva, O.A., and Golubenko, M.V., Syntropic genes and the cardiovascular continuum, Inf. Vestn. Vavilovskogo O-va. Genet. Sel., 2006, vol. 10, no. 3, pp. 479—491.
Puzyrev, V.P. and Freidin, M.B., Genetic view on the phenomenon of combined diseases in man, Acta Nat., 2009, vol. 1, no. 3, pp. 52—57.
Gottesman, O., Drill, E., Lotay, V., et al., Can genetic pleiotropy replicate common clinical constellations of cardiovascular disease and risk?, PLoS One, 2012, vol. 7. e46419. https://doi.org/10.1371/journal.pone.0046419
Puzyrev, V.P., Genetic bases of human comorbidity, Russ. J. Genet., 2015, vol. 51, no. 4, pp. 408—417. https://doi.org/10.1134/S1022795415040092
Rankinen, T., Sarzynski, M.A., Ghosh, S., and Bouchard, C., Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors?, Circ. Res., 2015, vol. 116, pp. 909—922. https://doi.org/10.1161/CIRCRESAHA.116.302888
Jia, X., Yang, Y., Chen, Y., et al., Multivariate analysis of genome-wide data to identify potential pleiotropic genes for type 2 diabetes, obesity and coronary artery disease using MetaCCA, Int. J. Cardiol., 2019, vol. 283, pp. 144—150. https://doi.org/10.1016/j.ijcard.2018.10.102
Tabarés-Seisdedos, R., Dumont, N., Baudot, A., et al., No paradox, no progress: inverse cancer comorbidity in people with other complex diseases, Lancet Oncol., 2011, vol. 12, pp. 604—608. https://doi.org/10.1016/S1470-2045(11)70041-9
Catalá-López, F., Suárez-Pinilla, M., Suárez-Pinilla, P., et al., Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577 013 participants of 50 observational studies, Psychother. Psychosom., 2014, vol. 83, pp. 89—105. https://doi.org/10.1159/000356498
Seo, J. and Park, M., Molecular crosstalk between cancer and neurodegenerative diseases, Cell Mol. Life Sci., 2020, vol. 77, pp. 2659—2680. https://doi.org/10.1007/s00018-019-03428-3
Houck, A.L., Seddighi, S., and Driver, J.A., At the crossroads between neurodegeneration and cancer: a review of overlapping biology and its implications, Curr. Aging Sci., 2018, vol. 11, pp. 77—89. https://doi.org/10.2174/1874609811666180223154436
Manolio, T.A., Collins, F.S., Cox, N.J., et al., Finding the missing heritability of complex diseases, Nature, 2009, vol. 461, pp. 747—753. https://doi.org/10.1038/nature08494
Claussnitzer, M., Cho, J.H., Collins, R., et al., A brief history of human disease genetics, Nature, 2020, vol. 577, pp. 179—189. https://doi.org/10.1038/s41586-019-1879-7
Dipple, K.M. and McCabe, E.R., Phenotypes of patients with simple Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics, Am. J. Hum. Genet., 2000, vol. 66, pp. 1729—1735. https://doi.org/10.1086/302938
Badano, J.L. and Katsanis, N., Beyond Mendel: an evolving view of human genetic disease transmission, Nat. Rev. Genet., 2002, vol. 3, pp. 779—789. https://doi.org/10.1038/nrg910
Sidransky, E., Heterozygosity for a Mendelian disorder as a risk factor for complex disease, Clin. Genet., 2006, vol. 70, pp. 275—282. https://doi.org/10.1111/j.1399-0004.2006.00688.x
Katsanis, N., The continuum of causality in human genetic disorders, Genome Biol., 2016, vol. 17, p. 233. https://doi.org/10.1186/s13059-016-1107-9
Blair, D.R., Lyttle, C.S., Mortensen, J.M., et al., A nondegenerate code of deleterious variants in Mendelian loci contributes to complex disease risk, Cell, 2013, vol. 155, pp. 70—80. https://doi.org/10.1016/j.cell.2013.08.030
Cohen, J., Pertsemlidis, A., Kotowski, I.K., et al., Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9, Nat. Genet., 2005, vol. 37, pp. 161—165. https://doi.org/10.1038/ng1509
Han, K., Holder, J.L.J., Schaaf, C.P., et al., SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties, Nature, 2013, vol. 503, pp. 72—77. https://doi.org/10.1038/nature12630
Auer, P.L., Teumer, A., Schick, U., et al., Rare and low-frequency coding variants in CXCR2 and other genes are associated with hematological traits, Nat. Genet., 2014, vol. 46, pp. 629—634. https://doi.org/10.1038/ng.2962
Lin, A., Ching, C.R.K., Vajdi, A., et al., Mapping 22q11.2 gene dosage effects on brain morphometry, J. Neurosci., 2017, vol. 37, pp. 6183—6199. https://doi.org/10.1523/JNEUROSCI.3759-16.2017
Wainschtein, P., Jain, D.P., Yengo, L., et al., Recovery of trait heritability from whole genome sequence data, BioRxiv, 2019. https://www.biorxiv.org/content/10.1101/588020v1.
Bezzina, C.R., Lahrouchi, N., and Priori, S.G., Genetics of sudden cardiac death, Circ. Res., 2015, vol. 116, pp. 1919—1936. https://doi.org/10.1161/CIRCRESAHA.116.304030
Bečanović, K., Nørremølle, A., Neal, S.J., et al., A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease, Nat. Neurosci., 2015, vol. 18, pp. 807—816. https://doi.org/10.1038/nn.4014
Corvol, H., Blackman, S.M., Boëlle, P.Y., et al., Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis, Nat. Commun., 2015, vol. 6, p. 8382. https://doi.org/10.1038/ncomms9382
Jordan, D.M., Frangakis, S.G., Golzio, C., et al., Identification of cis-suppression of human disease mutations by comparative genomics, Nature, 2015, vol. 524, pp. 225—229. https://doi.org/10.1038/nature14497
Castel, S.E., Cervera, A., Mohammadi, P., et al., Modified penetrance of coding variants by cis-regulatory variation contributes to disease risk, Nat. Genet., 2018, vol. 50, pp. 1327—1334. https://doi.org/10.1038/s41588-018-0192-y
Holmans, P. and Stone, T., Using genomic data to find disease-modifying loci in Huntington’s disease (HD), Methods Mol. Biol., 2018, vol. 1780, pp. 443—461. https://doi.org/10.1007/978-1-4939-7825-0_20
Kim, K.H., Hong, E.P., Shin, J.W., et al., Genetic and functional analyses point to FAN1 as the source of multiple Huntington disease modifier effects, Am. J. Hum. Genet., 2020, vol. 107, pp. 96—110. https://doi.org/10.1016/j.ajhg.2020.05.012
Posey, J.E., Harel, T., Liu, P., et al., Resolution of disease phenotypes resulting from multilocus genomic variation, N. Engl. J. Med., 2017, vol. 376, pp. 21—31. https://doi.org/10.1056/NEJMoa1516767
Lupski, J.R., Belmont, J.W., Boerwinkle, E., et al., Clan genomics and the complex architecture of human disease, Cell, 2011, vol. 147, pp. 32—43. https://doi.org/10.1016/j.cell.2011.09.008
Gibson, G., Rare and common variants: twenty arguments, Nat. Rev. Genet., 2012, vol. 13, pp. 135—145. https://doi.org/10.1038/nrg3118
Gaugler, T., Klei, L., Sanders, S.J., et al., Most genetic risk for autism resides with common variation, Nat. Genet., 2014, vol. 46, pp. 881—885. https://doi.org/10.1038/ng.3039
Dean, M., Approaches to identify genes for complex human diseases: lessons from Mendelian disorders, Hum. Mutat., 2003, vol. 22, pp. 261—274. https://doi.org/10.1002/humu.10259
Peltonen, L., Perola, M., Naukkarinen, J., et al., Lessons from studying monogenic disease for common disease, Hum. Mol. Genet., 2006, vol. 15, no. 1, pp. R67—R74. https://doi.org/10.1093/hmg/ddl060
Scheuner, M.T., Yoon, P.W., and Khoury, M.J., Contribution of Mendelian disorders to common chronic disease: opportunities for recognition, intervention, and prevention, Am. J. Med. Genet., Part C, 2004, vol. 125, pp. 50—65. https://doi.org/10.1002/ajmg.c.30008
Kathiresan, S., Willer, C.J., Peloso, G.M., et al., Common variants at 30 loci contribute to polygenic dyslipidemia, Nat. Genet., 2009, vol. 41, pp. 56—65. https://doi.org/10.1038/ng.291
van der Harst, P., van Setten, J., Verweij, N., et al., 52 Genetic loci influencing myocardial mass, J. Am. Coll. Cardiol., 2016, vol. 68, pp. 1435—1448. https://doi.org/10.1016/j.jacc.2016.07.729
Freund, M.K., Burch, K.S., Shi, H., et al., Phenotype-specific enrichment of Mendelian disorder genes near GWAS regions across 62 complex traits, Am. J. Hum. Genet., 2018, vol. 103, pp. 535—552. https://doi.org/10.1016/j.ajhg.2018.08.017
Spataro, N., Rodríguez, J.A., Navarro, A., et al., Properties of human disease genes and the role of genes linked to Mendelian disorders in complex disease aetiology, Hum. Mol. Genet., 2017, vol. 26, pp. 489—500. https://doi.org/10.1093/hmg/ddw405
Jin, W., Qin, P., Lou, H., et al., A systematic characterization of genes underlying both complex and Mendelian diseases, Hum. Mol. Genet., 2012, vol. 21, pp. 1611—1624. https://doi.org/10.1093/hmg/ddr599
Van, D.P., Veldink, J.H., Van, B.M., et al., Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2, Neurology, 2011, vol. 76, pp. 2066—2072. https://doi.org/10.1212/WNL.0b013e31821f445b
Lattante, S., Pomponi, M.G., Conte, A., et al., ATXN1 intermediate-length polyglutamine expansions are associated with amyotrophic lateral sclerosis, Neurobiol. Aging, 2018, vol. 64, pp. 157.e1—157.e5. https://doi.org/10.1016/j.neurobiolaging.2017.11.011
Choubtum, L., Witoonpanich, P., Kulkantrakorn, K., et al., Trinucleotide repeat expansion of TATA-binding protein gene associated with Parkinson’s disease: a Thai multicenter study, Parkinsonism Relat. Disord., 2016, vol. 28, pp. 146—149. https://doi.org/10.1016/j.parkreldis.2016.05.008
Vasil’ev, V.B., Geneticheskie osnovy mitokhondrial’nykh boleznei (Genetic Basis of Mitochondrial Diseases), Nestor-Istoriya, 2006.
Sulaiman, S.A., Rani, Z.M., Radin, F.Z.M., et al., Advancement in the diagnosis of mitochondrial diseases, J. Transl. Genet. Genom., 2020, vol. 4, pp. 159—187.
Kang, D. and Hamasaki, N., Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer, Curr. Med. Chem., 2005, vol. 12, pp. 429—441. https://doi.org/10.2174/0929867053363081
Veronese, N., Stubbs, B., Koyanagi, A., et al., Mitochondrial genetic haplogroups and cardiovascular diseases: data from the Osteoarthritis Initiative, PLoS One, 2019, vol. 14, no. 3. e0213656. https://doi.org/10.1371/journal.pone.0213656
Wallace, D.C. and Chalkia, D., Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease, Cold Spring Harb. Perspect. Biol., 2013, vol. 5, no. 11, p. a021220. https://doi.org/10.1101/cshperspect.a021220
Boyle, E.A., Li, Y.I., and Pritchard, J.K., An expanded view of complex traits: from polygenic to omnigenic, Cell, 2017, vol. 169, pp. 1177—1186. https://doi.org/10.1016/j.cell.2017.05.038
Melamed, R.D., Emmett, K.J., Madubata, C., et al., Genetic similarity between cancers and comorbid Mendelian diseases identifies candidate driver genes, Nat. Commun., 2015, vol. 6, p. 7033. https://doi.org/10.1038/ncomms8033
ClinGen. https://clinicalgenome.org.
Ingles, J., Goldstein, J., Thaxton, C., et al., Evaluating the clinical validity of hypertrophic cardiomyopathy genes, Circ. Genom. Precis. Med., 2019, vol. 12, no. 2. e002460. https://doi.org/10.1161/CIRCGEN.119.002460
Alimohamed, M.Z., Johansson, L.F., Posafalvi, A., et al., Diagnostic yield of targeted next generation sequencing in 2002 Dutch cardiomyopathy patients, Int. J. Cardiol., 2021, vol. 332, pp. 99—104. https://doi.org/10.1016/j.ijcard.2021.02.069
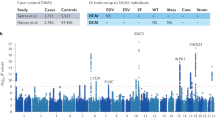
Tadros, R., Francis, C., Xu, X., et al., Shared genetic pathways contribute to risk of hypertrophic and dilated cardiomyopathies with opposite directions of effect, Nat. Genet., 2021, vol. 53, pp. 128—134. https://doi.org/10.1038/s41588-020-00762-2
Wooten, E.C., Hebl, V.B., Wolf, M.J., et al., Formin homology 2 domain containing 3 variants associated with hypertrophic cardiomyopathy, Circ. Cardiovasc. Genet., 2013, vol. 6, pp. 10—18. https://doi.org/10.1161/CIRCGENETICS.112.965277
Harper, A.R., Goel, A., Grace, C., et al., Common genetic variants and modifiable risk factors underpin hypertrophic cardiomyopathy susceptibility and expressivity, Nat. Genet., 2021, vol. 53, pp. 135—142. https://doi.org/10.1038/s41588-020-00764-0
Villard, E., Perret, C., Gary, F., et al., A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy, Eur. Heart J., 2011, vol. 32, pp. 1065—1076. https://doi.org/10.1093/eurheartj/ehr105
Meder, B., Rühle, F., Weis, T., et al., A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy, Eur. Heart J., 2014, vol. 35, pp. 1069—1077. https://doi.org/10.1093/eurheartj/eht251
Esslinger, U., Garnier, S., Korniat, A., et al., Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy, PLoS One, 2017, vol. 12, no. 3. e0172995. https://doi.org/10.1371/journal.pone.0172995
MendelVar. https://mendelvar.mrcieu.ac.uk/.
GTEx Portal. https://gtexportal.org/home/.
Verweij, N., Benjamins, J.W., Morley, M.P., et al., The genetic makeup of the electrocardiogram, Cell Syst., 2020, vol. 11, pp. 229—238. https://doi.org/10.1016/j.cels.2020.08.005
UCSC genome browser. http://genome.ucsc.edu/.
Pirruccello, J.P., Bick, A., Wang, M., et al., Analysis of cardiac magnetic resonance imaging in 36 000 individuals yields genetic insights into dilated cardiomyopathy, Nat. Commun., 2020, vol. 11, p. 2254. https://doi.org/10.1038/s41467-020-15823-7
Garnier, S., Harakalova, M., Weiss, S., et al., Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23, Eur. Heart J., 2021, vol. 42, pp. 2000—2011. https://doi.org/10.1093/eurheartj/ehab030
Diets, I.J., Prescott, T., Champaigne, N.L., et al., A recurrent de novo missense pathogenic variant in SMARCB1 causes severe intellectual disability and choroid plexus hyperplasia with resultant hydrocephalus, Genet. Med., 2019, vol. 21, pp. 572—579. https://doi.org/10.1038/s41436-018-0079-4
Wieczorek, D., Bögershausen, N., Beleggia, F., et al., A comprehensive molecular study on Coffin Siris and Nicolaides Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling, Hum. Mol. Genet., 2013, vol. 22, no. 25, pp. 5121—5135. https://doi.org/10.1093/hmg/ddt366
Radio, F.C., Pang, K., Ciolfi, A., et al., SPEN haploinsufficiency causes a neurodevelopmental disorder overlapping proximal 1p36 deletion syndrome with an episignature of X chromosomes in females, Am. J. Hum. Genet., 2021, vol. 108, pp. 502—516. https://doi.org/10.1016/j.ajhg.2021.01.015
Zollino, M., Marangi, G., Ponzi, E., et al., Intragenic KANSL1 mutations and chromosome 17q21.31 deletions: broadening the clinical spectrum and genotype—phenotype correlations in a large cohort of patients, J. Med. Genet., 2015, vol. 52, pp. 804—814. https://doi.org/10.1136/jmedgenet-2015-103184
Rattka, M., Westphal, S., Gahr, B.M., et al., Spen deficiency interferes with Connexin 43 expression and leads to heart failure in zebrafish, J. Mol. Cell Cardiol., 2021, vol. 155, pp. 25—35. https://doi.org/10.1016/j.yjmcc.2021.01.006
León, L.E., Benavides, F., Espinoza, K., et al., Partial microduplication in the histone acetyltransferase complex member KANSL1 is associated with congenital heart defects in 22q11.2 microdeletion syndrome patients, Sci. Rep., 2017, vol. 7, p. 1795. https://doi.org/10.1038/s41598-017-01896-w
Yang, M., Zhang, Y., and Ren, J., Acetylation in cardiovascular diseases: molecular mechanisms and clinical implications, Biochim. Biophys. Acta, Mol. Basis Dis., 2020, vol. 1866, p. 165836. https://doi.org/10.1016/j.bbadis.2020.165836
Backs, J. and Olson, E.N., Control of cardiac growth by histone acetylation/deacetylation, Circ. Res., 2006, vol. 98, pp. 15—24. https://doi.org/10.1161/01.RES.0000197782.21444.8f
Bick, A.G., Flannick, J., Ito, K., et al., Burden of rare sarcomere gene variants in the Framingham and Jackson Heart Study cohorts, Am. J. Hum. Genet., 2012, vol. 91, pp. 513—519. https://doi.org/10.1016/j.ajhg.2012.07.017
Watanabe, K., Stringer, S., Frei, O., et al., A global overview of pleiotropy and genetic architecture in complex traits, Nat. Genet., 2019, vol. 51, pp. 1339—1348. https://doi.org/10.1038/s41588-019-0481-0
Broekema, R.V., Bakker, O.B., and Jonkers, I.H., A practical view of fine-mapping and gene prioritization in the post-genome-wide association era, Open Biol., 2020, vol. 10, p. 190221. https://doi.org/10.1098/rsob.190221
Cano-Gamez, E. and Trynka, G., From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases, Front. Genet., 2020, vol. 11, p. 424. https://doi.org/10.3389/fgene.2020.00424
Bekker, O.B., Claringbould, A., Westra, H.-J., et al., Linking common and rare disease genetics through gene regulatory networks, medRxive, 2021. https://doi.org/10.1101/2021.10.21.21265342
King, E.A., Davis, J.W., and Degner, J.F., Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval, PLoS Genet., 2019, vol. 15, no. 12. e1008489. https://doi.org/10.1371/journal.pgen.1008489
Funding
This work was carried out within the State Task of the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by D. Novikova
Rights and permissions
About this article
Cite this article
Nazarenko, M.S., Sleptcov, A.A. & Puzyrev, V.P. “Mendelian Code” in the Genetic Structure of Common Multifactorial Diseases. Russ J Genet 58, 1159–1168 (2022). https://doi.org/10.1134/S1022795422100052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422100052