Abstract
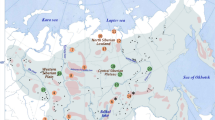
A panel of 15 microsatellite loci was used to study the modern structure of the sable gene pool in the southern part of the range. A high level of population differentiation was determined. The existence of several population clusters of the sable was revealed on the vast territory from Western Siberia to Transbaikalia. This part of the species range is distinguished by a high number of sables; another peculiarity is that the territories allowed for hunting alternate with a significant number of reserve territories. The abilities to identify individuals by population and regional affiliations were determined. In the study, the reliability of the prediction of population belonging (SCOR coefficient) of individuals to the pooled sample was estimated; the estimate showed that the accuracy (the number of correctly identified individuals) and reliability of identification significantly increases on average for each individual with an increase in the number of loci from 8 to 15. The results of the study complement the reference database of genetic data of populations of geographic regions of the species and make it possible to solve a number of problems associated with hunting on the territories bordering the nature reserves, which contributes to the preservation of intraspecific diversity of the sable.




Similar content being viewed by others
REFERENCES
Timofeev, V.V. and Nadeev, V.N., Sobol’ (Sable), Moscow: Zagotizdat, 1955.
Monakhov, G.I. and Bakeev, N.N., Sobol’ (Sable), Moscow: Lesnaya Promyshlenost’, 1981, 2nd ed.
Bazhanov, V.S., A new subspecies of sable from Altai, Trudy Kazakhskii filiala AN SSSR za 1942 god (Proceedings of the Kazakh Branch of the USSR Academy of Sciences for 1942), Alma-Ata: Akad. Nauk KazSSR, 1943, pp. 53–54.
Kuznetsov, B.A., Martes zibellina L., 1758–sable, in Mlekopitayushchie Kazakhstana (Mammals of Kazakhstan), Moscow: Mosk. O-vo Ispyt. Prir., 1948.
Lobachev, Yu.S. and Afanas’ev, Yu.G., Sable, Martes zibellina Linnaeus, 1758, in Mlekopitayushchie Kazakhstana (Mammals of Kazakhstan), Alma-Ata: Akad. Nauk KazSSR, 1982, vol. 3, part 2, pp. 101–119.
Monakhov, V.G., Size structure of the sable in the Lake Baikal region: a decadal analysis over the last sixty years, Biol. Bull., 2014, no. 1, pp. 47–54. https://doi.org/10.1134/S1062359014010063
Pavlov, M.P., Korsakova, I.B., Timofeev, V.V., and Safonov, V.G., Akklimatizatsiya okhotnich’e-promyslovykh zverei i ptits v SSSR (Acclimatisation of Commercial Game Mammals and Birds in the Soviet Union), Kirov: Volgo-Vyatskoe Knizhnoe Izd., 1973.
Kashtanov, S.N., Svishcheva, G.R., Pishchulina, S.L., et al., Geographical structure of the sable (Martes zibellina L.) gene pool on the basis of microsatellite loci analysis, Russ. J. Genet., 2015, vol. 51, no. 1, pp. 69–79. https://doi.org/10.1134/S1022795415010044
Kashtanov, S.N., Stolpovsky, Y.A., Meshchersky, I.G., et al., Taxonomic status and genetic identification of Altai sable (Martes zibellina averini Bazhanov, 1943), Russ. J. Genet., 2018, vol. 54, no. 11, pp. 1342–1351. https://doi.org/10.1134/S1022795418110078
Chernikin, E.M., Ekologiya sobolya (Martes zibellina Lunnaeus, 1758) v Barguzinskom zapovednike (Ecology of Sable (Martes zibellina Lunnaeus, 1758) in Barguzinsky Reserve), Ulan-Ude: Buryat. Gos. Univ., 2006.
Aspi, J., Roininen, E., Kiiskila, J., et al., Genetic structure of the northwestern Russian wolf populations and gene flow between Russia and Finland, Conserv. Genet., 2009, vol. 10, pp. 815–826.
Michaux, J.R., Hardy, O.J., Justy, F., et al., Conservation genetics and population history of the threatened European mink Mustela lutreola, with an emphasis on the west European population, Mol. Ecol., 2005, vol. 14, pp. 2373–2388.
Pertoldi, C., Breyne, P., Cabria, M.T., et al., Genetic structure of the European polecat (Mustela putorius) and its implication for conservation strategies, J. Zool., 2006, vol. 270, pp. 102–105.
Colli, L., Cannas, R., Deiana, A.M., et al., Microsatellite variability of Sardinian pine martens, Martes martes, Zool. Sci., 2011, vol. 28, pp. 580–586.
O’Shea, K., Genetic study of recent samples of American marten (Martes americana) from Vermont, UVM Honors College Senior Theses, 2014, vol. 6.
Davis, C.S. and Strobeck, C., Isolation, variability, and cross-species amplification of polymorphic microsatellite loci in the family Mustelidae, Mol. Ecol., 1998, vol. 7, no. 12, pp. 1776–1778.
Fleming, M.A., Ostrander, E.A., and Cook, J.A., Microsatellite markers for American mink (Mustela vison) and ermine (Mustela erminea), Mol. Ecol., 1999, vol. 8, no. 8, pp. 1352–1355.
Natali, C., Banchi, E., Ciofi, C., et al., Characterization of 13 polymorphic microsatellite loci in the European pine marten Martes martes, Conserv. Genet. Resour., 2010, vol. 2, pp. 397–399.
Kashtanov, S.N., Afanasiev, K.I., Potapov, S.G., and Lazebny, O.E., Microsatellite analysis of two captive populations of sable (Martes zibellina L.), Russ. J. Genet., 2011, vol. 47, no. 12, pp. 1438–1443.
R Development Core Team, R: A Language and Environment for Statistical Computing, Vienna: R Foundation for Statistical Computing, 2014.
Jombart, T., Adegenet: an R package for the multivariate analysis of genetic markers, Bioinformatics, 2008, vol. 24, no. 11, pp. 1403–1405.
Adamack, A.T. and Gruber, B., PopGenReport: simplifying basic population genetic analyses, Methods Ecol. Evol., 2014, vol. 5, no. 4, pp. 384–387.
Keenan, K., McGinnity, Ph., Cross, T.F., et al., DiveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors, Methods Ecol. Evol., 2013, vol. 4, no. 8, pp. 782–788.
Botstein, D., White, R.L., Skolnick, M.H., and Davis, R.W., Construction of a genetic linkage map in man using restriction fragment length polymorphisms, Am. J. Hum. Genet., 1980, vol. 32, pp. 314–331.
Gruber, B. and Adamack, A.T., Landgenreport: a new R function to simplify landscape genetic analysis using resistance surface layers, Mol. Ecol. Resour., 2015, vol. 15, no. 5, pp. 1172–1178.
Nei, M., Genetic distance between populations, Am. Nat., 1972, vol. 106, no. 949, pp. 283–292.
Kamvar, Z.N., Tabima, J.F., and Grünwald, N.J., Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction, Peer J., 2014, vol. 2. e281. https://doi.org/10.7717/peerj.281
Rannala, B. and Mountain, J.L., Detecting immigration by using multilocus genotypes, Proc. Acad. Natl. Acad. Sci. U.S.A., 1997, vol. 94, pp. 9197–9201.
Piry, S., Alapetite, A., Cornuet, J.M., Paetkau, D., Baudouin, L., and Estoup, A., GENECLASS2: a software for genetic assignment and first-generation migrant detection, J. Hered., 2004, vol. 95, pp. 536–539.
Excoffier, L., Laval, G., and Schneider, S., Arlequin ver. 3.0: an integrated software package for population genetics data analysis, Evol. Bioinf. Online, 2007, vol. 1, pp. 47–50.
Wahlund, S., Zusammensetzung von Populationen und Korrelationserscheinungen vom Standpunkt der Vererbungslehre aus betrachtet, Hereditas, 1928, vol. 11, no. 1, pp. 65–106.
Wright, S., The genetical structure of populations, Ann. Eugen., 1951, vol. 15, pp. 323–354.
Wright, S., The interpretation of population structure by F-statistics with special regard to systems of mating, Evolution, 1965, vol. 19, pp. 395–420.
Jost, L., GST and its relatives do not measure differentiation, Mol. Ecol., 2008, vol. 17, pp. 4015–4026.
Hedrick, P.W., A standardized genetic differentiation measure, Evolution, 2005, vol. 59, pp. 1633–1638.
Jost, L., Reply: D vs G'ST: response to Heller and Siegismund (2009) and Ryman and Leimar (2009), Mol. Ecol., 2009, vol. 18, pp. 2088–2091.
Kronholm, I., Loudet, O., and de Meaux, J., Influence of mutation rate on estimators of genetic differentiation–lessons from Arabidopsis thaliana, BMC Genet., 2010, vol. 11, p. 33. https://doi.org/10.1186/1471-2156-11-33
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Kashtanov, S.N., Shitova, M.V., Somova, M.M. et al. Identification of Sable (Martes zibellina L.) Populations in the Southern Part of the Species Range. Russ J Genet 57, 1179–1188 (2021). https://doi.org/10.1134/S1022795421100057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795421100057