Abstract
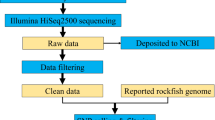
Northern snakehead Ophiocephalus argus Cantor is an endemic freshwater species in China. This species is widely distributed in the lower Yellow and Yangtze river systems in eastern China. In the present study, six cDNA libraries of Ophiocephalus argus were sequenced using Illumina HiSeq4000 paired-end sequencing technology. 67 564 sequences from 79 500 964 paired-end reads were generated. A total of 7182 unigenes were clustered into 25 functional categories by searching against the COG database, and BLAST2GO search assigned 33 710 unigenes to 59 GO terms. From the 67 564 assembled unique sequences, 21 464 SSRs were identified in 14 997 unique sequences. Among the 7992 with compound formation (SSRs), 100 primer pairs were successfully designed for further validation, and 42 of them produced polymorphic products in Ophiocephalus argus individuals. The number of alleles per locus ranged from 5 to 19, the observed and expected heterozygosity estimates ranged from 0.178 to 0.985 and from 0.421 to 0.929, respectively. The generation of such larges cale sequence data in the present study provides a valuable resource for gene discovery and molecular marker development.

Similar content being viewed by others
REFERENCES
Jabeen, M. and Afzal, M., Densitometric analysis of serum transferrins in two air-breathing fish (Channidae: Channiformes), Asian J. Biochem., 2010, vol. 5, no. 4, pp. 214—221.
Xiao, M. S., Cui, F., Kang, J., and Zhang, X.H., Genetic structure and variation of wild Ophicephalus argus Cantor from Huaihe River based on mtDNA D-loop sequences. J. Huazhong Nor. Univ.(Nat. Sci.), 2013, vol. 47, no. 1, pp. 82—90 (in Chinese).
Wu, X.W., Economy Fauna of China, Beijing: Science Press, 1979.
Liu, J., Cui, Y., and Liu, J., Resting metabolism and heat increment of feeding in mandarin fish (Siniperca chuatsi) and Chinese snakehead (Channa argus), Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2000, vol. 127, pp. 131—138.
Lou, L., A test on the culture of Channa argus, Freshwater Fish.,1995, no. 5, pp. 38—40 (in Chinese).
Wu, L. F., Zhang, D. M., Huang, Q., and Liu, C.L., The food analysis, population resources and utilization of snakehead fish (Ophiocephelus argus) in Huanghua Lake, J. Jilin Agric. Univ., 2000, vol. 22, no. 3, pp. 104—106 (in Chinese).
Yu, H.Y., Ou, Y.S., Wu, X.P., Dai, Y.G., and Zou, S.Y., Studies on age and growth of Ophiocephalus argus in Poyang Lake, Life Sci. Res., 2008, vol. 12, no. 1, pp. 91—94 (in Chinese).
Landis, G.A.M., Lapointe, N.W.R., and Angermeier, P.L., Individual growth and reproductive behavior in a newly established population of northern snakehead (Channa argus), Potomac River, U.S.A., Hydrobiologia, 2011, vol. 661, pp. 123—131.
Li, J.W., Biological characteristics and artificial culture techniques of Ophiocephalus argus, China Fish., 1999, no. 10, pp. 28—31.
Huang, Z.P., The culture technique of Channa argus in pond, J. Aquacult., 2011, no. 2, pp. 37—38 (in Chinese).
Zhang, X.Q., Gong, S.Y., Liu, J., He, X.G., and Gao, J.H., Studies on age and growth of Channa argus in the Wanghu Lake, Acta Hydrobiol. Sin., 1999, vol. 23, no. 6, pp. 600—603.
Nie, G.X., Fu, Y.R., Zhang, H., and Wei, X.J., The analysis of nutrition components in the muscle of Channa argus,Freshwater Fish., 2002, vol. 32, no. 2, pp. 46—47 (in Chinese).
Qin, W., Ni, J.G., Zhang, X.B., and Zhou, J.F., Comparative studies on chromosomes in the snakehead fish Channa argus and Ophiocephalidae,Reservoir Fish., 2004, vol. 24, no. 5, pp. 14—16 (in Chinese).
Yin, W.L. and Dai, J.H., Studies on the isozymes of different tissues in Ophiocephalus argus, J. Huazhong Nor. Univ.(Nat. Sci.), 2000, vol. 20, no. 3, pp. 17—20 (in Chinese).
Li, X., Ou, Y.S., Wu, X.P., Dai, Y.G., Hua, Y.Q., and Jiao, L., Studies on the EST and LDH isozymes in different tissues of Ophicephalus argus in Poyang Lake, HubeiAgric. Sci., 2010, vol. 49, no. 1, pp. 153—155.
Zhuo, X.L., Liang, R.S., Chen, Y.F., Huang, G.J., Yu, D.H., and Zou, J.X., Genetic characterization of northern snakehead (Channa argus) populations in China using microsatellite markers, Biochem. Syst. Ecol., 2012, vol. 43, pp. 25—31.
Li, D.Y., Kang, D.H., Yin, Q.Q., Sun, X.W., and Liang, L.Q., Microsatellite DNA marker analysis of genetic diversity in wild common carp (Cyprinus carpio L.) populations, J. Genet. Genomics, 2007, vol. 34, no. 11, pp. 984—993.
Xiao, M.X., Xia, H., and Bao, F., Isolation and characterization of 15 microsatellite loci for Ophicephalus argus Cantor, Russ. J. Genet., 2015, vol. 51, no. 10, pp. 1044—1047.
Li, H.J., Liu, M., Ye, S., and Yang, F., De novo assembly, gene annotation, and molecular marker development using Illumina paired-end transcriptome sequencing in the clam Saxidomus purpuratus,Genes Genom., 2017, vol. 39, no. 6, pp. 675—685.
Núñez-Acuña, G. and Gallardo-Escárate, C., Identification of immune-related SNPs in the transcriptome of Mytilus chilensis through high-throughput sequencing, Fish Shellfish Immun., 2013, vol. 35, pp. 1899—1905.
Li, H.J., Zhang, J.J., Li, H.B., Gao, X.G., and He, C.B., Development and characterization of 13 polymorphic microsatellite markers for the Chinese surf clam (Mactra chinensis) through Illumina pairedend sequencing, Conserv. Genet. Resour., 2014, no. 6, pp. 877—879.
Grabherr, M. G., Haas, B. J., Yassour, M., et al., Full-length transcriptome assembly from RNA-Seq data without a reference genome, Nat. Biotechnol., 2011, vol. 29, no. 7, pp. 644—652.
Franceschini, A., Szklarczyk, D., Frankild, S., Kuhn, M., Simonovic, M., Roth, A., Lin, J., Minguez, P., Bork, P., von Mering, C., and Jensen, L.J., STRING v9.1: protein—protein interaction networks, with increased coverage and integration, Nucleic Acids Res., 2013, vol. 41, pp. D808—D815.
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., and Madden, T.L., BLAST+: architecture and applications, BMC Bioinf., 2009, no. 10, p. 421.
Conesa, A., Gotz, S., Garcia-Gomez, J.M., Terol, J., Talon, M., and Robles, M., Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research, Bioinformatics, 2005, vol. 21, pp. 3674—3676.
Thiel, T., Michalek, W., Varshney, R.K., and Graner, A.A., Exploiting EST databases for the development and characterization of gene derived SSR-markers in barley (Hordeum vulgare L.), Theor. Appl. Genet., 2003, no. 10, pp. 411—422.
Bassam, B.J., Caetano-Anolles, G., and Gresshoff, P.M., Fast and sensitive silver staining of DNA in polyacrylamide gels, Anal. Biochem., 1991, vol. 196, pp. 80—83.
Rousset, F., Genepop’007: a complete re-implementation of the Genepop software for Windows and Linux, Mol. Ecol. Resour., 2008, no. 8, pp. 103—106.
Yeh, F.C. and Yang, R.C., POPGENE version 1.31, Microsoft Window-Based Freeware for Population Genetic Analysis, Edmonton: University of Alberta, 1999. http://microsoft.com/softlib/ slfiles/hpgl.exe.
Xu, P., Zhang, X.F., Wang, X.M., Li, J.T., Liu, G.M., Kuang, Y.Y., et al., Genome sequence and genetic diversity of the common carp, Cyprinus carpio,Nat. Genet., 2014, vol. 46, pp. 1212—1219.
Howe, K., Clark, M.D., Torroja, C.F., et al., The zebrafish reference genome sequence and its relationship to the human genome, Nature, 2013, vol. 496, pp. 498—503.
Li, D.J., Deng, Z., Qin, B., Liu, X.H., and Men, Z.H., De novo assembly and characterization of bark transcriptome using Illumina sequencing and development of EST-SSR markers in rubber tree (Hevea brasiliensis Muell. Arg.), BMC Genomics, 2012, no. 13, pp. 192—206.
Ma, K., Qiu, G., Feng, J., and Li, J., Transcriptome analysis of the oriental river prawn, Macrobrachium nipponense using 454 pyrosequencing for discovery of genes and markers, PLoS One, 2012, vol. 7, no. 6. e39727
Liao, X., Cheng, L., Xu, P., Lu, G., Wachholtz, M., Sun, X.W., and Chen, S.L., Transcriptome analysis of Crucian Carp (Carassius auratus), an important aquaculture and hypoxia-tolerant species, PLoS One, 2013, vol. 8, no. 4. e62308
Li, Y.H., Wang, H.P., Yao, H., O’Bryant, P., Rapp, D., Guo, L., and Waly, E.A., De novo transcriptome sequencing and analysis of male, pseudo-male and female yellow perch, Perca flavescens,PLoS One, 2017, vol. 12, no. 2. e0171187
Wang, L.H., Zhang, Y.X., Qi, X.Q., Gao,Y., and Zhang, X.R., Development and characterization of 59 polymorphic cDNA-SSR markers for the edible oil crop Sesamum indiwcum (Pedaliaceae), Am. J. Bot., 2012, no. 10, pp. e394—e398. http://www.amjbot.org/content/99/10/e394.full-aff-1.
ACKNOWLEDGMENTS
We would like to thank the anonymous reviewers for the helpful comments. This research was supported by the Natural Science Foundation of Anhui Province, China (1708085MC78), Anhui Province Aquaculture Industry Technology System (Sciences and Technology education department, Anhui Provincial Agriculture Commission, China (2016 [84]), Anhui Province Key Research and Development Plan Project, China (1804a070200119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. Mingsong Xiao, Fangyin Bao, Yan Zhaoand Jixiang He declare that they have no conflict of interests.
Statement on the welfare of animals. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Xiao, M., Bao, F., Zhao, Y. et al. Development of Genetic Novel SSR Markers by Transcriptome Sequencing in Ophicephalus argus Cantor. Russ J Genet 56, 253–260 (2020). https://doi.org/10.1134/S1022795420020143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420020143