Abstract
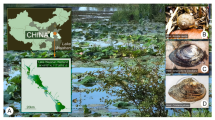
The structure of telomeric repeat (TTAGGG) n was determined and the length of telomeric DNA (tDNA) was measured in three species of gastropods from the family Benedictiidae that are endemic to Lake Baikal. Fluorescence in situ hybridization (FISH) confirmed the localization of a telomeric repeat at the chromosome ends. The sizes of tDNA in “giant” eurybathic, psammo-pelobiontic species Benedictia fragilis and shallow water litho-psammobiontic species B. baicalensis with medium shell sizes were similar (16 ± 2.9 and 15 ± 2.1 kb, respectively), but they had a greater length than that of the shallow water spongio-litobiontic species Kobeltocochlea martensiana with small shells (10.5 ± 1.5 kb). We discuss tendencies in age-related changes in tDNA length in snails and a possible mechanism for maintaining tDNA size in ontogeny.
Similar content being viewed by others
References
Traut, W., Szczepanowski, M., Vitkova, M., et al., The telomere repeat motif of basal Metazoa, Chromosome Res., 2007, vol. 15, pp. 371–382.
Samassekou, O., Gadji, M., Drouin, R., and Yan, J., Sizing the ends: normal length of human telomeres, Ann. Anat., 2010, vol. 192, pp. 284–291.
Zhdanova, N.S., Minina, Yu.M., Karamysheva, T.V., et al., The structure of long telomeres in chromosomes of the Iberian shrew, Russ. J. Genet., 2010, vol. 46, no. 9, pp. 1084–1086.
Gomes, N., Ryder, O., Houck, M., et al., Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination, Aging Cell, 2011, vol. 10, pp. 761–768.
Koroleva, A.G., Evtushenko, E.V., Timoshkin, O.A., et al., Telomeric DNA length and phylogenetic relationship of Baikal and Siberian planarians (Turbellaria, Tricladida), Cell Tissue Biol., 2013, vol. 55, no. 4, pp. 369–374.
Seluanov, A., Chen, Z., Hine, C., et al., Telomerase activity coevolves with body mass, not lifespan, Aging Cell, 2007, vol. 6, pp. 45–52.
Gorbunova, V. and Seluanov, A., Coevolution of telomerase activity and body mass in mammals: from mice to beavers, Mech. Ageing Dev., 2009, vol. 130, pp. 3–9.
Haussmann, M., Winkler, D., O’Reilly, K., et al., Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones, Proc. R. Soc. Lond., 2003, vol. 270, pp. 1387–1392.
Landman, N., Cochran, J., Cerrato, R., et al., Habitat and age of the giant squid (Architeuthis sanctipauli) inferred from isotopic analyses, Mar. Biol., 2004, vol. 144, pp. 685–691.
Rawat, R., Anatomy of Mollusca, Daryaganj: International Scientific Publishing Academy (ISPA), 2010, p. 9.
Lukas, J., The biology, exploitation, and mariculture of giant clams (Tridacnidae), Rev. Fish. Sci., 1994, vol. 2, no. 3, pp. 181–223.
Munro, D. and Blier, P., The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes, Aging Cell, 2012, vol. 11, no. 5, pp. 845–855.
Sosnowska, D., Richardson, C., Sonntag, W., et al., A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living non-colonial animal, J. Gerontol. Biol. Sci. Med. Sci., 2013. doi 10.1093/gerona/glt201
Estabrooks, S., The possible role of telomeres in the short life span of the bay scallop, Argopecten irradians irradians (Lamark 1819), J. Shellfish Res., 2007, vol. 26, no. 2, pp. 307–313.
Godwin, R., Brown, I., Montgomery, S., et al., Telomere dynamics in the Sydney rock oyster (Saccostrea glomerata): an investigation into the effects of age, tissue type, location and time of sampling, Mar. Biol., 2012, vol. 159, pp. 77–86.
Gruber, H., Schaible, R., Ridgway, I., et al., Telomere-independent ageing in the longest-lived non-colonial animal, Arctica islandica, Exp. Gerontol., 2014, vol. 51, pp. 38–45.
Nomoto, Y., Hirai, M., and Ueshima, R., Cloning of molluscan telomere DNA with (TTAGGG)n repeat and its chromosomal location in the freshwater snail Biwamelania habei, Zool. Sci., 2001, vol. 18, pp. 417–422.
Plohl, M., Prats, E., Martinez-Lage, A., et al., Telomeric localization of the vertebrate-type hexamer repeat, (TTAGGG)n, in the wedgeshell clam Donax trunculus and other marine invertebrate genomes, Biol. Chem., 2002, vol. 277, no. 22, pp. 19839–19846.
Wang, Y. and Guo, X., Chromosomal mapping of the vertebrate telomeric sequence (TTAGGG)n in four bivalve molluscs by fluorescence in situ hybridization, J. Shellfish Res., 2001, vol. 20, pp. 1187–1190.
Vitturi, R., Gianguzza, P., Colomba, M., et al., Cytogenetics in the sacoglossan Oxynoe olivacea (Mollusca: Opisthobranchia): karyotype, chromosome banding and fluorescent in situ hybridization, Mar. Biol., 2000, vol. 137, no. 4, pp. 577–582.
Vitturi, R., Colomba, M., Gianguzza, P., and Pirrone, A., Chromosomal location of ribosomal DNA (rDNA), (GATA)n and (TTAGGG)n telomeric repeats in the neogastropod Fasciolaria lignaria (Mollusca: Prosobranchia), Genetics, 2000, vol. 108, pp. 253–257.
Vitturi, R., Libertini, A., Sineo, L., et al., Cytogenetics of the land snails Cantareus asperses and C. mazzullii (Mollusca: Gastropoda: Pulmonata), Micron, 2005, vol. 36, pp. 351–357.
Guo, X. and Allen, S., Fluorescence in situ hybridization of vertebrate telomere sequence to chromosome ends of the Pacific oyster Crassostrea gigas Thunberg, J. Shellfish Res., 1997, vol. 16, no. 1, pp. 87–89.
Pérez-García, C., Guerra-Varela, J., Morán, P., and Pasantes, J., Chromosomal mapping of rRNA genes, core histone genes and telomeric sequences in Brachidontes puniceus and Brachidontes rodriguezi (Bivalvia, Mytilidae), BMC Genet., 2010, vol. 11, no. 109, pp. 1–9.
Colomba, M., Vitturi, R., Castriota, L., et al., Fish mapping of 18S-28S and 5S ribosomal DNA, (GATA)n and (TTAGGG)n telomeric repeats in the periwinkle Melarhaphe neritoides (Prosobranchia, Gastropoda, Caenogastropoda), Heredity, 2002, vol. 88, pp. 381–384.
Cross, I., Diaz, E., Sanchez, I., and Rebordinos, L., Molecular and cytogenetic characterization of Crassostrea angulata chromosomes, Aquaculture, 2005. doi 10.1016/j.aquaculture.2005.02.039
Carrilho, J., Perez-Garcia, C., Leitao, A., et al., Cytogenetic characterization and mapping of rDNAs, core histone genes and telomeric sequences in Venerupis aurea and Tapes rhomboids (Bivalvia: Veneridae), Genetics, 2011, vol. 139, pp. 823–831.
Kozhov, I., Lake Baikal and Its Life, The Hague: Junk, 1963.
Vadeboncoeur, Y., McIntyre, P., and Vander Zanden, M., Borders of biodiversity: life at the edge of the world’s large lakes, BioScience, 2011, vol. 61, no. 7, pp. 526–537.
Sitnikova, T. and Shirokaya, A., New data on deepwater Baikal limpets found in hydrothermal vents and oil-seeps, Arch. Molluskenkd., 2013, vol. 42, no. 2, pp. 257–278.
Sitnikova, T.Ya., Prosobranch gastropods (Gastropoda: Prosobranchia) of Baikala: morphology, taxonomy, biology, and fauna formation, Doctoral (Biol.) Dissertation, Irkutsk: Limnological Institute, Siberian Branch of Russian Academy of Sciences, 2004.
Maximova, N. and Sitnikova, T., Size, age and sex ratio in Maackia herderiana (Gerstfeldt, 1859) (Gastropoda: Caenogastropoda: Baicaliidae) from south Baikal Lake, Ruthenica, 2006, vol. 16, nos. 1–2, pp. 97–104.
Gorbushin, A.M., Structure of winter growth interruption lines and mechanism of their formation on Hydrobia ulvae Pennant 1777 (Gastropoda, Prosobranchia) shell in the White Sea, Zool. Zh., 1993, vol. 72, no. 11, pp. 29–34.
Kozminskii, E.V., Growth, population demographic structure, and age determination in Bithynia tentaculata (Gastropoda, Prosobranchia), Zool. Zh., 2003, vol. 82, no. 5, pp. 567–576.
Frydrychova, R., Grossmann, P., Truba, P., et al., Phylogenetic distribution of TTAGG telomeric repeats in insects, Genome, 2004, vol. 47, pp. 163–178.
Ijdo, J., Wells, R., Baldini, A., and Reeders, S., Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR, Nucleic Acids Res., 1991, vol. 19, no. 17, p. 4780.
Joffe, B., Solovei, I., and Macgregor, H., Ordered arrangement and rearrangement of chromosomes during spermatogenesis in two species of Planarians (Plathelminthes), Chromosoma, 1998, vol. 107, pp. 173–183.
Sasaki, T. and Fujiwara, H., Detection and distribution patterns of telomerase activity in insects, Eur. J. Biochem., 2000, vol. 267, pp. 3025–3031.
Biessmann, H., Kobeski, F., Walter, M., et al., DNA organization and length polymorphism at the 2l telomeric region of Anopheles gambiae, Insect Mol. Biol., 1998, vol. 7, no. 1, pp. 83–93.
Poberezhnyi, E.S. and Sitnikova, T.Ya., Chromosomes of Baikal mollusk Benedictia baikalensis (Gastropoda, Prosobranchia), Zool. Zh., 1978, vol. 5, no. 8, pp. 1270–1273.
Pomazkova, G.I., Novoe v izuchenii flory i fauny Baikala i ego basseina: Sbornik nauchnykh trudov (News on the Study of the Flora and Fauna of Baikal and Its Basin: A Collection of Scientific Papers), Irkutsk: Irkutsk Gos. Univ., 1988.
Anchelin, M., Murcia, L., Alcaraz-Perez, F., et al., Behavior of telomere and telomerase during aging and regeneration in zebrafish, PLoS One, 2011, vol. 6, no. 2. e16955. doi 10.1371/journal.pone.0016955
Allshire, R., Dempster, M., and Hastie, N., Human telomeres contain at least three types of G-rich repeat distributed non-randomly, Nucleic Acids Res., 1989, vol. 17, no. 12, pp. 4611–4627.
Brown, W., MacKinnon, P., Villasant, A., et al., Structure and polymorphism of human telomere-associated DNA, Cell, 1990, vol. 63, no. 1, pp. 119–132.
Graakjaer, J., Bischoff, C., Korsholm, L., et al., The pattern of chromosome-specific variations in telomere length in humans is determined by inherited, telomerenear factors and is maintained throughout life, Mech. Ageing Dev., 2003, vol. 124, no. 5, pp. 629–640.
Graakjaer, J., Der-Sarkissian, H., Schmitz, A., et al., Allele-specific relative telomere lengths are inherited, Hum. Genet., 2006, vol. 119, pp. 344–350.
Horn, T., Robertson, B., and Gemmell, N., The use of telomere length in ecology and evolutionary biology, Heredity, 2010, vol. 105, pp. 497–506.
Zemskaya, T., Sitnikova, T., Kiyashko, S., et al., Faunal communities at sites of gas- and oil-bearing fluids in Lake Baikal, J. Geophys. Res., 2012, vol. 32, no. 5, pp. 437–451.
Francis, N., Gregg, T., Owen, R., et al., Lack of ageassociated telomere shortening in long- and short-lived species of sea urchins, FEBS Lett., 2006, vol. 580, pp. 4713–4717.
Jacob, N., Stout, A., and Price, C., Modulation of telomere length dynamics by the subtelomeric region of Tetrahymena telomeres, Mol. Biol. Cell, 2004, vol. 15, pp. 3719–3728.
Jemielity, S., Kimura, M., Parker, K., et al., Short telomeres in short-lived males: what are the molecular and evolutionary causes?, Aging Cell, 2007, vol. 6, pp. 225–233.
Tan, T., Rahman, R., Jaber-Hijazi, F., et al., Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms, Proc. Natl. Acad. Sci. U.S.A., 2012. www.pnas.org/cgi/doi/10.1073/pnas.1118885109.
Koziol, C., Borojevic, R., Steffen, R., and Müller, W., Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: the telomerase activity in somatic cells, Mech. Ageing Dev., 1998, vol. 100, no. 2, pp. 107–120.
Zielke, S. and Bodnar, A., Telomeres and telomerase activity in scleractinian corals and Symbiodinium spp., Biol. Bull., 2010, vol. 218, pp. 113–121.
Lang, G., Wang, Y., Nomura, N., and Matsumura, M., Detection of telomerase activity in tissues and primary cultured lymphoid cells of Penaeus japonicas, Mar. Biotechnol., 2004, vol. 6, pp. 347–354.
Magnenat, L., Tobler, H., and Müller, F., Developmentally regulated telomerase activity is correlated with chromosomal healing during chromatin diminution in Ascaris sum, Mol. Cel. Biol., 1999, vol. 19, no. 5, pp. 3457–3465.
Owen, R., Sarkis, S., and Bodnar, A., Developmental pattern of telomerase expression in the sand scallop, Euvola ziczac, Invertebr. Biol., 2007, vol. 126, pp. 40–45.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.G. Koroleva, E.V. Evtushenko, N.V. Maximova, A.V. Vershinin, T.Y. Sitnikova, S.V. Kirilchik, 2015, published in Genetika, 2015, Vol. 51, No. 3, pp. 362–370.
Rights and permissions
About this article
Cite this article
Koroleva, A.G., Evtushenko, E.V., Maximova, N.V. et al. Length and structure of telomeric DNA in three species of Baikal gastropods (Caenogastropoda: Hydrobioidea: Benedictiidae). Russ J Genet 51, 300–307 (2015). https://doi.org/10.1134/S1022795415030060
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795415030060