Abstract
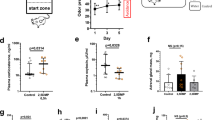
The review considers stress as a physiological state of the organism, affecting the cellular, genomic, and population levels. Literature data and cytogenetic studies by the author support basic statements of the physiological hypothesis of mutation, which was advanced as early as in the 1940s. Studies of pheromonal effects in germline and somatic cells of the house mouse demonstrated the role of olfactory stressors in generating genetic variation in microevolutionary changes.
Similar content being viewed by others
References
Selye, H., Syndrome Produced by Diverse Nocuous Agents, Nature, 1936, vol. 138, p. 32.
Waisel, Y., Epilogue, Bielefeld. Ökol. Beitr., 1992, vol. 6, p. 115.
Selye, H., The Story of the Adaptation Syndrome, Montreal: Acta Medical, 1952.
Selye, H. Na urovne tselogo organizma (At the Level of the Whole Body), Moscow: Nauka, 1972.
Selye, H., Stress without Distress, New York: New American Library, 1974.
Selye, H., The Physiology and Pathology of Exposure to Stress, Montreal: Acta Medical, 1950, p. 203.
Arshavskii, I.A., Mechanisms and Characteristics of Physiological and Pathological Stress at Different Ages, in Aktual’nye problemy stressa (Current Problems of Stress), Kishinev: Shtiintsa, 1976, pp. 5–23.
Kakhana, M.S., Mel’nik, B.E., and Robu, A.I., The Role of Hypothalamic Endocrine Interrelations during Variable Stress Reactions, in Aktual’nye problemy stressa (Current Problems of Stress), Kishinev: Shtiintsa, 1976, pp. 115–124.
Pogodaev, K.I., On the Biological Basis of “Stress” and “Adaptation Syndrome,” in Aktual’nye problemy stressa (Current Problems of Stress), Kishinev: Shtiintsa, 1976, pp. 211–229.
Sovremennye kontseptsii evolyutsionnoi genetiki (Current Concepts of Evolutionary Genetics), Shumnyi, V.K. and Markel’, A.L., Eds., Novosibirsk: Inst. Tsitologii i Genetiki, Sib. Otd., Ross. Akad.Nauk, 2000.
Osnovy neiroendokrinologii (Basics of Neuroendocrinology), Shalyapina, V.G. and Shabanov, P.D., Eds., St. Petersburg: Elbi-SPb, 2005.
Stress-Inducible Processes in Higher Eukaryotic Cells, Koval, T.M., Ed., New York: Plenum, 1997.
Nikulina, E.M., Miczek, K.A., and Hammer, R.P., Prolonged Effects of Repeated Social Defeat Stress on mRNA Expression and Function of m-Opioid Receptors in the Ventral Tegmental Area of Rats, Neuropsychopharmacology, 2005, vol. 30, no. 6, pp. 1096–1103.
Margulis, B.A. and Guzhova, I.V., Stress Proteins in Eukaryotic Cell, Tsitologiya, 2000, vol. 42, no. 4, pp. 323–342.
Soti, C., Pal, C., Papp, B., and Csermely, P., Molecular Chaperones as Regulatory Elements of Cellular Networks, Curr. Opin. Cell Biol., 2005, vol. 17, no. 2, pp. 210–215.
Baraboi, V.A., Brekhman, I.I., Golotin, V.G., and Kudryashov, Yu.B., Perekisnoe okislenie i stress (Peroxide Oxidation and Stress), St. Petersburg: Nauka, 1992.
Clem, R.J., Apoptosis as a Stress Response: Lessons from an Insect Virus, in Stress-Inducible Processes in Higher Eukaryotic Cells, Koval, T.M., Ed., New York: Plenum, 1997, pp. 109–136.
Schuler, M. and Green, D.R., Mechanisms of p53-Dependent Apoptosis, Biochem. Soc. Trans., 2001, vol. 29, no. 6, pp. 684–688.
Ben-Porath, I. and Weinberg, R.A., The Signals and Pathways Activating Cellular Senescence, Int. J. Biochem. Cell Biol., 2005, vol. 37, no. 5, pp. 961–976.
Liu, Y.F., Bertram, K., Perides, G., et al., Stress Induces Activation of Stress-Activated Kinases in the Mouse Brain, J. Neurochem., 2004, vol. 89, pp. 1034–1043.
McClintock, B., The Significance of Responses of the Genome to Challenge, Science, 1984, vol. 226, no. 4 676, pp. 792–801.
Velkov, V.V., New Insights into the Molecular Mechanisms of Evolution: Stress Increases Genetic Diversity, Izv. Akad. Nauk SSSR, Ser. Biol., 2002, vol. 36, no. 2, pp. 277–286.
de La, Serna I.L., Carlson, K.A., Hill, D.A., et al., Mammalian SWI-SNF Complexes Contribute To Activation of the hsp70 Gene, Mol. Cell Biol., 2000, vol. 8, pp. 2839–2851.
Rahman, I., Marwick, J., and Kirkham, P., Redox Modulation of Chromatin Remodeling: Impact on Histone Acetylation and Deacetylation, NF-KappaB and Pro-Inflammatory Gene Expression, Biochem. Pharmacol., 2004, vol. 68, no. 6, pp. 1255–1267.
Zhan, Q., Gadd45a, a P53-and BRCA1-Regulated Stress Protein, in Cellular Response to DNA Damage, Mutat. Res., 2005, vol. 569, nos. 1–2, pp. 133–143.
Liu, J., Wang, X., Shigenaga, M.K., et al., Immobilization Stress Causes Oxidative Damage to Lipid, Protein, and DNA in the Brain of Rats, FASEB J., 1996, vol. 10, pp. 1532–1538.
Limoli, C.L., Hartmann, A., Shephard, L., et al., Apoptosis, Reproductive Failure, and Oxidative Stress in Chinese Hamster Ovary Cells with Compromised Genomic Integrity, Cancer Res., 1998, vol. 58, pp. 3712–3718.
Twigg, J., Fulton, N., Gomez, E., et al., Analysis of the Impact of Intracellular Reactive Oxygen Species Generation on the Structural and Functional Integrity of Human Spermatozoa: Lipid Peroxidation, DNA Fragmentation and Effectiveness of Antioxidants, Hum. Reprod., 1998, vol. 13, pp. 1429–1436.
Borodin, P.M. and Belyaev, D.K., Effect of Stress on Frequency of Crossingover in the 2nd Chromosome of Domestic Mouse, Dokl. Akad. Nauk SSSR, 1980, vol. 253, no. 3, pp. 727–729.
Dyuzhikova, N.A., Savenko, Yu.N., Vaido, A.I., and Lopatina, N.G., Changes in Heterochromatin Conformation Play a Triggering Role in Long-Term Modification in Hippocampal Neurons of Rats with Different Characteristics of the Nervous System Excitability during Post-traumatic Stress, Ekol. Kul’tura Obr., 2003, nos. 10–11, pp. 67–68.
Opol’skii, A.F., The Influence of Hypothalamus on the Mutagenesis in Animal Somatic Cells, in Chuvstvitel’nost’ organizmov k mutagennym faktoram i vozniknovenie mutatsii (Sensitivity of Organisms to Mutagenic Factors and the Appearence of Mutations), Vilnius: Izd. Vilnius Gos. Univ., 1982, pp. 20–21.
Seredenin, S.B., Durnev, A.D., and Vedernikova, A.A., The Effect of Emotional Stress on the Chromosome Aberration Frequency in Mouse Bone Marrow Cells, Byull. Exp. Biol. Med., 1980, no. 7, pp. 91–92.
Fischman, H.K. and Kelly, D.D., Chromosomes and Stress, Int. J. Neurosci., 1999, vol. 99, pp. 201–219.
Fischman, H.K., Pero, R.W., and Kelly, D.D., Psychogenic Stress Induces Chromosomal and DNA Damage, Int. J. Neurosci., 1996, vol. 84, pp. 219–227.
Hunt, C.R., Sim, J.E., Sullivan, S.J., et al., Genomic Instability and Catalase Gene Amplification Induced by Chronic Exposure to Oxidative Stress, Cancer Res., 1998, vol. 58, pp. 3986–3992.
Umegaki, K., Higuchi, M., Inoue, K., and Esashi, T., Influence of One Bout of Intensive Running on Lymphocyte Micronucleus Frequencies in Endurance-Trained and Untrained Men, Int. J. Sports Med., 1998, vol. 19, pp. 581–585.
Ingel, F.I., Perspectives of Micronuclear Test Application to Human Blood Lymphocytes, Cultivated in the Condition of Cytokinetic Block, Ekol. Genet., 2006, vol. 4, no. 3, pp. 7–19.
Kaidanov, L.Z., Genetika populyatsii (Genetics of Populations), Moscow: Vysshaya Shkola, 1996.
Schmidt, A.L. and Anderson, L.M., Repetitive DNA Elements as Mediators of Genomic Change in Response to Environmental Cues, Biol. Rev., 2006, vol. 81, pp. 531–543.
Lobashev, M.E., Physiological (Paranecrotic) Hypothesis of the Mutation Process, Vestn. Leningr. Univ., 1947, no. 8, pp. 10–29.
Kerkis, Yu.Ya. and Skorova, S.V., On the Factors, Controlling Intensity of Spontaneous Mutation Process, Inf. Byull. Nauchn. Sov. Probl. Radiobiol., 1977, no. 20, pp. 51–52.
Lieber, M.M., Environmentally Responsive Mutator Systems: Toward a Unifying Perspective, Rev. Biol., 1998, vol. 91, pp. 425–457.
Zakharov, I.K., Yurchenko, N.N., Ivannikov, A.V., et al., Mutation Outbreaks and Transposons in Natural Populations of Drosophila melanogaster, Inf. Vestn. VOGiS, 2001, no. 16, pp. 10–12.
Menichini, P., Viaggi, S., Gallerani, E., et al., A Gene Trap Approach to Isolate Mammalian Genes Involved in the Cellular Response to Genotoxic Stress, Nucleic Acids Res., 1997, vol. 25, pp. 4803–4807.
Shackelford, R.E., Kaufmann, W.K., and Paules, R.S., Cell Cycle Control, Checkpoint Mechanisms, and Genotoxic Stress, Env. Health Persp., 1999, vol. 107, pp. 5–24.
Scolnick, D.M. and Halazonetis, T.D., Chfr Defines a Mitotic Stress Checkpoint That Delays Entry into Metaphase, Nature, 2000, vol. 406, pp. 430–435.
Naumenko, E.V., Osadchuk, A.V., Serova, L.I., and Shishkina, G.T., Genetiko-fiziologicheskie mekhanizmy regulyatsii funktsii semennikov (Genetic Physiological Mechanisms of Regulation of Testicle Function), Novosibirsk: Nauka, 1983.
Hendrichs, H., On Social Stress in Mammals, Bielefeld. Okol. Beitr., 1992, vol. 6, pp. 105–110.
Breckle, S.-W., Salinity-Stress and Salt Recreation in Plants, Bielefeld. Ökol. Beitr., 1992, vol. 6, pp. 39–52.
Kruuk, L.E., Clutton-Brock, T.H., Albon, S.D., et al., Population Density Affects Sex Ratio Variation in Red Deer, Nature, 1999, vol. 399, pp. 459–461.
Christian, J.J., The Adrenalo-Pituitary System and Population Cycles in Mammals, J. Mammal., 1950, vol. 31, pp. 247–259.
Christian, J.J., Phenomena Associated with Population Density, Proc. Natl. Acad. Sci. USA, 1961, vol. 47, pp. 428–449.
Christian, J.J., Population Density and Reproductive Efficiency, Biol. Reprod., 1971, vol. 4, pp. 248–294.
Chitty, D., Population Processes in Vole and Their Relevance to General Theory, Can. J. Zool., 1960, vol. 38, pp. 99–113.
Chitty, D., The Natural Selection of Self-Regulating Behaviour in Animal Populations, Proc. Ecol. Soc. Aust., 1967, vol. 2, pp. 51–78.
Lee, A.K. and McDonald, I.R., Stress and Population Regulation in Small Mammals, Oxford Rev. Reprod. Biol., 1985, vol. 7, pp. 261–304.
Kerkis, Yu.Ya., Physiological Changes in the Cell as a Cause of Mutation, Usp. Sovrem. Biol., 1940, no. 1, pp. 344–350.
Khromov-Borisov, N.N., Physiological Theory of Mutation Process a Quarter of a Century Later, in Issledovaniya po genetike (Research in Genetics), Leningrad: Leningr. Gos. Univ., 1976, issue 6, pp. 16–31.
Kamyshev, N.G., Savvateeva, E.V., and Ponomarenko, V.V., About Neurohormonal Factors in Regulation of Genetic and Cytogenetic Processes, in Fiziologicheskaya genetika i genetika povedeniya (Physiological Genetics and Behavioral Genetics), Leningrad: Nauka, 1981.
Lobashev, M.E., Ponomarenko, V.V., Polyanskaya, G.G., and Tsapygina, R.I., On the Role of the Nervous System in Regulating a Variety of Genetic and Cytogenetic Processes, Zh. Evol. Biokhim. Fiziol., 1973, vol. 9, no. 4, pp. 396–406.
Lopatina, N.G., Ponomarenko, V.V., and Smirnova, G.P., Hypothesis of Nervous Regulation of the Process of Genetic Information Realization, in Problemy vysshei nervnoi deyatel’nosti i neirofiziologii (Problems of Higher Nervous Activity and Neurophysiology), Leningrad: Nauka, 1975, pp. 107–121.
Ponomarenko, V.V., Behavioral Genetics, in Fiziologicheskaya genetika (Physiological Genetics), Leningrad: Meditsina, 1976, pp. 350–382.
Tsapygina, R.I., The Study of Induced Chromosome Aberrations in the Mouse Cornea Epithelium in Connection with Functional State of Cerebral Cortex and Mitotic Diurnal Rhythm, in Issledovaniya po genetike (Research in Genetics), Leningrad: Leningr. Gos. Univ., 1974, issue 5, pp. 13–18.
Pimenova, M.N., The Effect of Neuroactive Substances on Cytogenetic Processes in Somatic Cells (Mouse and Human), Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrad: Leningr. Gos. Univ., 1975.
Polyanskaya, G.G., The Effect of Sympatectomy on Cytogenetic Processes in the Mice, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrad: Leningr. Gos. Univ., 1971.
Tsapygina, R.I., The Study of the Role of Central Nervous System in the Regulation of Cytogenetic Processes by Means of Conditioned Reflex Method, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrad: Leningr. Gos. Univ., 1972.
Kerkis, Yu.Ya., Osetrova, G.D., Loginova, V.V., et al., Genetic and Physiological (Humoral) Factors, Controlling the Induced and Spontaneous Mutation Process in Mammals, in Problemy teoreticheskoi i prikladnoi genetiki (Problems in Theoretical and Applied Genetics), Novosibirsk, 1973, pp. 75–94.
Vaido, A.I., Dyuzhikova, N.A., Shiryaeva, N.V., et al., Epigenetic Mechanisms of Long-Term Stress Effects, in Genetika v XXI veke: sovremennoe sostoyanie i perspektivy razvitiya (Genetics in the 21st Century: Current State and Prospects of Development) (Proc. III Conference All-Russian Society of Geneticists and Breeders), Moscow, 2004, vol. 1, p. 10.
Tsapygina, R.I., Daev, E.V., and Novikov, S.N., The Effect of Exogenous Metabolites on Cytokinesis in Generative Tissue of Laboratory Animals, in Issledovanie biologicheskogo deistviya antropogennykh faktorov, zagryaznyayushchikh vodoemy (Study of the Biological Effect of Anthropogenic Pollutants of Water Bodies), Irkutsk: Izd. Irkutsk Gos. Univ., 1979, pp. 157–162.
Belyaev, D.K., Destabilizing Selection as a Factor of Variation under Animal Domestication, Priroda, 1979, no. 2, pp. 36–45.
Belyaev, D.K. and Borodin, P.M., The Influence of Stress on Genetic Variation and Its Role in Evolution, in Evolyutsionnaya genetika (Evolutionary Genetics), Leningrad: Leningr. Gos. Univ., 1982, pp. 35–59.
Bruce, H.M., An Exteroceptive Block to Pregnancy in the Mouse, Nature, 1959, vol. 184, p. 105.
Southwick, C.H., Eosinophil Response of C57Br Mice to Behavioral Disturbance, Ecology, 1959, vol. 40, pp. 156–157.
Novikov, S.N., Feromony i Razmnozhenie Mlekopitayushchikh (Pheromones and Reproduction in Mammals), Leningrad: Nauka, 1988.
Brown, R.E., Rodents I: Effects of Odours on Reproductive Physiology (Primer Effects), in Social Odours in Mammals, Brown, R.E. and MacDonald D.W., Eds., Oxford: Claredon, 1985, vol. 1, pp. 245–344.
Jin, B.K., Franzen, L., and Baker, H., Regulation of C-Fos mRNA and fos Protein Expression in Olfactory Bulbs from Unilaterally Odor-Deprived Adult Mice, Int. J. Dev. Neurosci., 1996, vol. 14, pp. 971–982.
Beynon, R.J. and Hurst, J.L., Urinary Proteins and the Modulation of Chemical Scents in Mice and Rats, Peptides, 2004, vol. 25, pp. 1553–1563.
Fiber, J.M. and Swann, J.M., Testosterone Differentially Influences Sex-Specific Pheromone-Stimulated Fos Expression in Limbic Regions of Syrian Hamsters, Horm. Behav., 1996, vol. 30, pp. 455–473.
Halem, H.A., Cherry, J.A., and Baum, M.J., Vomeronasal Neuroepithelium and Forebrain Fos Responses to Male Pheromones in Male and Female Mice, J. Neurobiol., 1999, vol. 39, pp. 249–263.
Dudley, C.A., Rajendren, G., and Moss, R.L., Signal Processing in the Vomeronasal System: Modulation of Sexual Behavior in the Female Rat, Crit. Rev. Neurobiol., 1996, vol. 10, pp. 265–290.
Dlusen, D.E., Ramirez, V.D., Carter, C.S., and Getz, L.L., Male Vole Urine Changes Luteinizing Hormone Releasing Hormone and Norepinephrine in Female Olfactory Bulb, Science, 1981, vol. 212, pp. 573–575.
Singer, A.G., Clancy, A.N., Macrides, F., et al., Chemical Properties of a Female Mouse Pheromone That Stimulates Gonadotropin Secretion in Males, Biol. Reprod., 1988, vol. 38, pp. 193–199.
Daev, E.V., Genetic Consequences of Olfactory Stresses in Mice, Doctoral (Biol.) Dissertation, St. Petersburg: St. Petersb. Gos. Univ., 2006.
Lawton, A.D. and Whitsett, J.M., Inhibition of Sexual Maturation by a Urinary Pheromone in Male Prairie Deer Mice, Horm. Behav., 1979, vol. 13, pp. 128–138.
Pavlova, M.B., Garina, I.A., Dyuzhikova, N.A., et al., Corticosteroid Response and Indolamine Content of the Mouse Brain under the Action of a Pheromone, Disrupting Spermatogenesis, Fiziol. Zh., 1989, vol. 75, pp. 138–142.
Marois, G., Inhibition of Nidation in Mice by Modification of the Environment and Pheromones: Re-Establishment by Prolactin and Thioproperazine, Ann. Endocrinol., 1982, vol. 43, pp. 41–52.
Surinov, B.P., Karpova, N.A., Isaeva, V.G., and Kulish, Yu.S., PostStress States and Communicative Disturbances of Immunity and Blood, Patol. Fiziol. Eksp. Terapiya, 2000, no. 4, pp. 9–11.
Surinov, B.P., Karpova, N.A., and Zhovtun, L.P., Olfactory Stress: Immunosupression Dynamics in Mice with Different Genotype, Immunologiya, 2004, no. 3, pp. 183–185.
Cocke, R., Moynihan, J.A., Cohen, N., et al., Exposure to Conspecific Alarm Chemosignals Alters Immune Responses in BALB/c Mice, Brain Behav. Immun., 1993, vol. 7, pp. 36–46.
Moynihan, J.A., Karp, J.D., Cohen, N., and Cocke, R., Alterations in Interleukin-4 and Antibody Production Following Pheromone Exposure: Role of Glucocorticoids, J. Neuroimmunol., 1994, vol. 54, pp. 51–58.
Silver, L.M., Mouse Genetics: Concepts and Applications, New York: Oxford Univ. Press, 1995.
Daev, E.V., The Effect of Exogenous Metabolites on Cytogenetic Characteristics of Spermatogenesis and Reproductive Function in the Male House Mouse, Cand. Sci. (Biol.) Dissertation, Leningrad, 1983.
Daev, E.V., Pheromonal Regulation of Genetic Processes: Research on the House Mouse (Mus musculus L.), Russ. J. Genet., 1994, vol. 30, no. 8, pp. 964–970.
Aref’ev, A.A. and Daev, E.V., Impaired Spermatogenesis in House Mouse after Exposure to Urinary Volatiles of Mature Males, in Atual’nye problemy sovremennoi biologii: Problemy reproduktsii, differentsirovki i funktsionirovaniya kletok (Current Problems of Modern Biology: Problems of Cell Reproduction, Differentiation, and Function), Available from VINITI, 1987, Moscow, no. 3107-B87.
Daev, E.V. and Dukel’skaya, A.V., The Effect of Female Mouse Pheromone 2,5-Dimethylpyrazine on the Reproductive Characteristics of Males S57VL/6 Mice, Ekol. Genet., 2004, vol. 2, no. 1, pp. 44–49.
Daev, E.V., Surinov, B.P., Dukel’skaya, A.V., et al., Immunological, Cytogenetic and Behavior Changes in Male Mice CBA and C57BL/6 Exposed to Pheromone, Zh. Evol. Biokhim. Fiziol., 2005, vol. 41, no. 4, pp. 44–49.
Lenski, R.E. and Mitler, I.E., The Directed Mutation Controversy and Neo-Darwinism, Science, 1993, vol. 259, pp. 188–194.
Tzapigina, R., Aref’ev, A., Sverdlova, O., and Daev, E., Pheromonal Regulation Hypothesis of the Space-Genetic Structure of the House Mouse (Mus musculus L.) Populations, in World Congress of Landscape Ecology, Ottawa: International Association for Landscape Ecology, 1991, p. 84.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Daev, 2007, published in Genetika, 2007, Vol. 43, No. 10, pp. 1299–1310.
Rights and permissions
About this article
Cite this article
Daev, E.V. Stress, chemocommunication, and the physiological hypothesis of mutation. Russ J Genet 43, 1082–1092 (2007). https://doi.org/10.1134/S102279540710002X
Received:
Issue Date:
DOI: https://doi.org/10.1134/S102279540710002X