Abstract
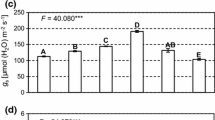
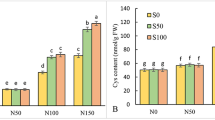
Productivity values, sodium accumulation in aboveground biomass, and photosynthetic indices of watercress (Nasturtium officinale) leaves were investigated under conditions resembling artificial closed ecological systems (CES). The seedlings were grown on nutrient media with various NaCl concentrations (0.7, 1.4, and 1.8 g/L) for 7, 14, and 19 days after transferring them to saline solutions. The productivity of plants on the seventh day of their growth on saline media did not differ from that of control plants. The decrease in plant productivity was noted in all the treatments starting from the 14th day after transferring the plants to saline solutions. When NaCl concentration in the nutrient solution was raised from 0.7 to 1.8 g/L, a significant increase in relative Na+ content in plant tissues was observed, regardless of the duration of NaCl treatment. A substantial decrease in chlorophyll (a + b) to carotenoid content ratio was noted on the seventh and 14th days in plants grown at 1.8 g/L NaCl. In plants treated for 7 days with 0.7 and 1.4 g/L NaCl, the content of chlorophylls a and b and carotenoids was found to increase, which indicates the tolerance of N. officinale to CES conditions. The relative content of chlorophylls a and b in the light-harvesting chlorophyll (a + b) complex was independent of the extent of salinity. The maximum quantum yield of photosystem II reaction in N. officinale plants had typically high values (Y(II)max of 0.755 ± 0.007). Using the Imaging Maxi version of the pulse amplitude-modulated (PAM) fluorometer, it was found that light curves for the effective quantum yield of photochemical and nonphotochemical fluorescence quenching (Y(II) and Y(NPQ), respectively) differed appreciably between the salt-treated and untreated plants in the case of long-term cultivation (19 days) at 0.7 and 1.4 g/L NaCl. The treatment with 1.8 g/L NaCl for the period from 14 to 19 days had no effect on light curves of Y(II) and Y(NPQ). It is argued that N. officinale can be used as a source of NaCl for humans under CES conditions.






Similar content being viewed by others
REFERENCES
Ushakova, S.A., Kovaleva, N.P., Gribovskaya, I.V., Dolgushev, V.A., and Tikhomirova, N.A., Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS, Adv. Space Res., 2005, vol. 36, p. 1349. https://doi.org/10.1016/j.asr.2004.09.017
Tikhomirova, N.A., Pavlova, A.M., Ushakova, S.A., Trifonov, S.V., Gribovskaya, I.V., and Tikhomirov, A.A., Yeild characteristics of green plants cultivated on liquid products of human expmetabolites processing for a bio-technical life support system, Aviakosm. Ekol. Med., 2017, vol. 51, p. 51. https://doi.org/10.21687/0233-528X-2017-51-1-51-57
Srivastava, A.K., Srivastava, S., Lokhande, V.H., D’Souza, S.F., Lokhande, V.H., and Suprasanna, P., Salt stress reveals differential antioxidant and energetics responses in glycophyte (Brassica juncea L.) and halophyte (Sesuvium portulacastrum L.), Front. Environ. Sci., 2015, vol. 3, p. 1. https://doi.org/10.3389/fenvs.2015.00019
Kitajima, M. and Butler, W.L., Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone, Biochim. Biophys. Acta, Bioenerg., 1975, vol. 376, p. 105. https://doi.org/10.1016/0005-2728(75)90209-1
Kreslavski, V.D., Carpentier, R., Klimov, V.V., Murata, N., and Allakhverdiev, S.I., Molecular mechanisms of stress resistance of the photosynthetic apparatus, Biochemistry (Moscow) Suppl. Ser. A: Membr. Cell Biol., 2007, vol. 1, p. 185.
Ushakova, S.A., Tikhomirov, A.A., Velichko, V.V., Golovko, T.K., Tabalenkova, G.N., Zakhozhii, I.G., and Matusevich, V.V., Comparison of the productivity of green crops—possible representatives of the link of higher plants of bioregenerative life support systems, Aviakosm. Ekol. Med., 2010, vol. 44, p. 42.
Anishchenko, O.V., Tolomeev, A.P., Ivanova, E.A., Drobotov, A.V., Kolmakova, A.A., Zuev, I.V., and Gribovskaya, I.V., Accumulation of elements by submerged (Stuckenia pectinate (L.) Börner) and emergent (Phragmites australis (Cav.) Trin. ex Steud.) macrophytes under different salinity levels, Plant Physiol. Biochem., 2020, vol. 154, p. 328. https://doi.org/10.1016/j.plaphy.2020.05.019
Wintermans, I.F.G.M. and De Mots, A., Spectrophotometric characteristics of chlorphylls a and b and their pheophytins in ethanol, Biochim. Biophys. Acta, Biophys. Incl. Photosynth., 1965, vol. 109, p. 448. https://doi.org/10.1016/0926-6585(65)90170-6
Lichtenthaler, H.K., Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, in Methods in Enzymology, Colowick, S.P. and Kaplan, N.O., Eds., San Diego: Academic, 1987, p. 350. https://doi.org/10.1016/0076-6879(87)48036-1
Genty, B., Briantais, J.M., and Baker, N.R., The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence, Biochim. Biophys. Acta, Gen. Subj., 1989, vol. 990, p. 87. https://doi.org/10.1016/S0304-4165(89)80016-9
Platt, T., Gallegos, C.L., and Harrison, W.G., Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton, J. Mar. Res., 1980, vol. 38, p. 687.
Tikhomirova, N.A., Influence of environmental factors on gas exchange and productivity of Salicornia europaea L. plants as a possible component of the phototrophic link in the life support system, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Tomsk: Tomsk State Univ., 2006.
Kramer, D.M., Johson, G., Kiirats, O., and Edwards, G., New fluorescence parameters for determination of QA redox state and excitation energy fluxes, Photosynth. Res., 2004, vol. 79, p. 209. https://doi.org/10.1023/B:PRES.0000015391.99477.0d
Robinson, S.R., John, W., Downton, S., and Millhouse, J.A., Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach, Plant Physiol., 1983, vol. 73, p. 238 https://doi.org/10.1104/pp.73.2.238
Guo, Y.-Y., Nie, H.-S., Yu, H.-Y. Kong, D.-S., and Wu, J.-Y., Effect of salt stress on the growth and photosystem II photochemical characteristics of Lycium ruthenicum Murr. seedlings, Photosynthetica, 2019, vol. 57, p. 564. https://doi.org/10.32615/ps.2019.068
Kaddour, R., Draoui, E., Baâtour, O. Mahmoudi, H., Tarchoune, I., Nasri, N., Gruber, M., and Lachaâl, M., Assessment of salt tolerance of Nasturtium officinale R. Br. using physiological and biochemical parameters, Acta Physiol. Plant., 2013, vol. 35, p. 3427. https://doi.org/10.1007/s11738-013-1377-8
Munns, R., Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses, Plant Cell Environ., 1993, vol. 16, p. 15. https://doi.org/10.1111/j.1365-3040.1993.tb00840.x
Genkel’, P.A., Fiziologiya zharo- i zasukhoustoichivosti rastenii (Physiology of Heat- and Drought Resistance of Plants), Moscow: Nauka, 1982.
Monirifar, H. and Barghi, M., Identification and selection for salt tolerance in alfalfa (Medicago sativa L.) ecotypes via physiological traits, Not. Sci. Biol., 2009, vol. 1, p. 63. https://doi.org/10.15835/nsb.1.1.3498
Hernández, J.A., Jiménez, A., Mullineaux, P., and Sevilla, F., Tolerance of pea plants (Pisum sativum) to long term salt stress is associated with induction of antioxidant defences, Plant Cell Environ., 2000, vol. 23, p. 853. https://doi.org/10.1046/J.1365-3040.2000.00602.x
Stepien, P. and Johnson, G.N., Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink, Plant Physiol., 2009, vol. 149, p. 1154. https://doi.org/10.1104/pp.108.132407
Belkhodja, R., Morales, F., Abadia, A., Gomez-Aparisi, J., and Abadia, J., Chlorophyll fluorescence as a possible tool for salinity tolerance screening in barley (Hordeum vulgare L.), Plant Physiol., 1994, vol. 104, p. 667. https://doi.org/10.1104/pp.104.2.667
Sharma, P., Jha, A.B., Dubey, R.S., and Pessarakli, M., Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions, J. Bot., 2012, vol. 2012, art. ID 217037. https://doi.org/10.1155/2012/217037
Souza, R.P., Machado, E.C., Silva, J.A.B., Lagoa, A.M.M.A., and Silveira, J.A.G., Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery, Environ. Exp. Bot., 2004, vol. 51, p. 45. https://doi.org/10.1016/S0098-8472(03)00059-5
Apse, M.P., Aharon, G.S., Snedden, W.A., and Blumwald, E., Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiporter in Arabidopsis, Science, 1999, vol. 285, p. 1256. https://doi.org/10.1126/science.285.5431.1256
Müller, M. and Santarius, K.A., Changes in chloroplast membrane lipids during adaptation of barley to extreme salinity, Plant Physiol., 1978, vol. 62, p. 326. https://doi.org/10.1104/pp.62.3.326
Zamknutaya sistema: chelovek–vysshie rasteniya (A Man–Higher Plants Closed System), Lisovskii, G.M., Ed., Novosibirsk: Nauka, 1979.
Funding
This work was supported by the fundamental research program of the Russian Academy of Sciences for 2013–2020, project no. 56.1.4 Sustainability of Higher Plant Cenoses Grown on Nutrient Media with Mineralized Organic Waste in Closed Human-Inhabited Ecological Systems.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by A. Bulychev
Abbreviations: AGB—aboveground biomass (dry matter content); Car—carotenoids; CES—closed ecological systems; Chl—chlorophyll; ETR—electron transport rate; LHC—light-harvesting chlorophyll (a + b) complex; PAR—photosynthetically active radiation; PFD—photon flux density; PSII— photosystem II; PSA—photosynthetic apparatus.
Rights and permissions
About this article
Cite this article
Pavlova, A.M., Gaevskii, N.A., Anishchenko, O.V. et al. Influence of NaCl on Productivity and Fluorescence Parameters of Nasturtium officinale R. Br. and Its Relevance to Artificial Closed Ecosystems. Russ J Plant Physiol 68, 1173–1185 (2021). https://doi.org/10.1134/S1021443721050137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721050137