Abstract
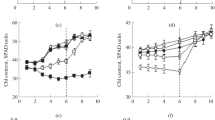
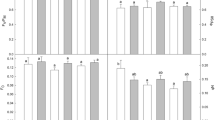
We investigated the response of chilling-sensitive plants of cucumber (Cucumis sativus L.) to prolonged permanent (6 days) (PLT) and short-term (3 h) daily exposures to low non-freezing temperatures (9 and 12°С) (DROP) lying on different sides of the critical value (10°С) corresponding to a phase transition of membrane lipids in chilling-sensitive plants from a liquid-crystalline to a solid gel structure. Effects and aftereffects of DROP treatments at temperatures of 9 and 12°С (DROP9 and DROP12, respectively) were identical. They caused a reduction in linear dimensions of plants (as compared with control plants) and enhanced chilling tolerance of leaves but did not influence photosynthetic activity and water relations. However, when exposure to these temperatures was permanent (PLT9 and PLT12), their effects on plants were different. PLT9 almost entirely suppressed plant growth and development, inactivated photosynthetic apparatus (PSA), increased relative electrolite leakage (REL), and boosted lipid peroxidation (LPO). PLT12 also considerably reduced plant height and leaf area and suppressed (but did not inactivate) PSA; it did not boost POL or increased REL. It is important that, after the termination of chilling, PLT9 plants (unlike PLT12 plants) could not quickly resume growth and restore photosynthetic activity. Thus, considerable differences in plant responses to various patterns of chilling (long permanent or short-term daily) are probably due to the fact that, in the case of DROP treatments, relatively short-term (3 h) chilling of plants is followed in the diurnal cycle by a prolonged period (21 h) of optimal temperature when possible deviations and/or disturbances of PSA are restored and toxic substances that could accumulate in the course of chilling metabolized and/or neutralized. Pronounced differences in plant response to permanent exposure to temperatures of 9 and 12°С probably depend on the fact that these temperatures lie on different sides of a critical value (10°С) below which chilling-sensitive plants suffer grave physiological disturbances due to cooling. In addition, we showed that different responses of plants to PLT and DROP treatments are not determined by a usual dose-effect relationship but depend in many respects on the pattern of temperature influence (prolonged or short-term, single or recurring). As a result, the number of DROP spans experienced by plants in the experiments proved to be more important than their duration (at least, within a time interval from 2 to 12 h).
Similar content being viewed by others
Abbreviations
- CT:
-
chilling tolerance
- DROP:
-
short-term daily drops of temperature
- MDA:
-
malonic dialdehyde
- PAR:
-
photosynthetically active radiation
- PLT:
-
permanent low temperature
- LPO:
-
lipid peroxidation
- PS:
-
photosystem
- PSA:
-
photosynthetic apparatus
- REL:
-
relative electrolyte leakage
- RWC:
-
relative water content
- TCA:
-
trichloroacetic acid
References
Salveit, M.E., Jr. and Morris, L.L., Overview on chilling injury of horticultural crops, in Chilling Injury of Horticultural Crops, Wang, C.Y., Ed., Boca Raton, FL: CRC, 1990, pp. 3–15.
Jones, H.G., Plant and Microclimate: A Quantitative Approach to Environmental Plant Physiology, Cambridge: Cambridge Univ. Press, 2014.
Lyons, J.M., Raison, J.K., and Steponkus, P.L., The plant membrane in response to low temperature: an overview, in Low Temperature Stress in Crop Plants: The Role of the Membrane, Lyons, J.M., Raison, J.K., and Graham, D., Eds., New York: Academic, 1979, pp. 1–4.
Wang, C.Y. and Baker, Y.E., Effects of two free radical scavengers and intermittent warming on chilling injury and polar lipid composition of cucumber and sweet pepper fruits, Plant Cell Physiol., 1979, vol. 20, no. 1, pp. 243–251.
Wang, C.Y., Physiological and biochemical responses of plants to chilling stress, Hort. Sci., 1982, vol. 17, no. 2, pp. 173–186.
Wang, C.Y., Approaches to reduce chilling injury of fruit and vegetables, Hortic. Rev., 1993, vol. 15, pp. 63–95.
Koscielniak, J. and Biesaga-Koscielniak, J., The effect of short warm breaks during chilling on water status, intensity of photosynthesis of maize seedlings and final grain yield, J. Agron. Crop Sci., 2000, vol. 184, no. 1, pp. 1–12.
Skrudlik, G., Baczek-Kwinta, R., and Koscielniak, J., The effect of short warm breaks during chilling on photosynthesis and of antioxidant enzymes in plants sensitive to chilling, J. Agron. Crop Sci., 2000, vol. 184, no. 4, pp. 233–240.
Moe, R., Using temperature to control plant height, FloraCulture Int., 1991, vol. 1, no. 2, pp. 26–27.
Heins, R.D. and Erwin, J.E., The history of DIF and the use of a morning temperature dip to control plant height, Minnesota Flower Growers Bull., 1991, vol. 40, no. 5, pp. 1–4.
Erwin, J.E. and Heins, R.D., Thermomorphogenic responses in stem and leaf development, Hort. Sci., 1995, vol. 30, no. 5, pp. 940–949.
Markovskaya, E.F., Sysoeva, M.I., and Sherudilo, E.G., Kratkovremennaya gipotermiya i rastenie (Short-Term Hypothermia and Plant), Petrozavodsk: Karel. Nauch. Tsentr, Ross. Akad. Nauk, 2013.
Drozdov, S.N., Kurets, V.K., and Titov, A.F., Termorezistentnost’ aktivno vegetiruyushchikh rastenii (Thermoresistance of Actively Vegetating Plants), Leningrad: Nauka, 1984.
Heath, R.L. and Packer, L., Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation, Arch. Biochem. Biophys., 1968, vol. 125, no. 1, pp. 189–198.
Drozdov, S.N., Budykina, N.P., Kurets, V.K., and Balagurova, N.I., Determination of plant resistance to frost, in Metody otsenki ustoichivosti rastenii k neblagopriyatnym usloviyam sredy (Methods for Assessing Plant Resistance to Adverse Environmental Conditions), Leningrad: Kolos, 1976, pp. 222–228.
Grishenkova, N.N. and Lukatkin, A.S., Determination of plant tissue resistance to abiotic stresses using the conductometric method, Povolzh. Ekol. Zh., 2005, no. 1, pp. 3–11.
Pardossi, A., Vernieri, P., and Tognoni, F., Involvement of abscisic acid in regulating water status in Phaseolus vulgaris L. during chilling, Plant Physiol., 1992, vol. 100, no. 3, pp. 1243–1250.
Gómez, I., Pérez-Rodríguez, E., Viñegla, B., Figueroa, F., and Karsten, U., Effects of solar radiation on photosynthesis, UV-absorbing compounds and enzyme activities of the green alga Dasycladus vermicularis from Southern Spain, J. Photochem. Photobiol. B.: Biol., 1998, vol. 47, pp. 46–57.
Ikkonen, E.N., Shibaeva, T.G., and Titov, A.F., Response of the photosynthetic apparatus in cucumber leaves to daily short-term temperature drops, Russ. J. Plant Physiol., 2015, vol. 62, no. 4, pp. 494–498.
Stavang, J.A., Pettersen, R.I., Wendell, M., Solhaug, K.A., Junttila, O., Moe, R., and Olsen, J.E., Thermoperiodic growth control by gibberellins does not involve changes in photosynthetic or respiratory capacities in pea, J. Exp. Bot., 2010, vol. 61, no. 4, pp. 1015–1029.
Klimov, S.V., Popov, V.N., and Trunova, T.I., The relationship between the cold tolerance of tomato and cucumber organs and photosynthesis, Russ. J. Plant Physiol., 2000, vol. 47, no. 4, pp. 435–440.
King, A.I., Reid, M.S., and Patterson, B.D., Diurnal changes in the chilling sensitivity of seedlings, Plant Physiol., 1982, vol. 70, no. 1, pp. 211–214.
Alscher, G., Rietze, E., and Wiebe, H.-J., Diurnal chilling of some vegetable crops, Biotronics, 1988, vol. 17, pp. 17–20.
Rietze, E. and Wiebe, H.-J., Diurnal rhythm of chilling sensitivity, Sci. Hort., 1989, vol. 38, pp. 231–237.
Grimstad, S.O., The effect of a daily low temperature pulse on growth and development of greenhouse cucumber and tomato plants during propagation, Sci. Hort., 1993, vol. 53, pp. 53–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.G. Shibaeva, E.G. Sherudilo, A.F. Titov, 2018, published in Fiziologiya Rastenii, 2018, Vol. 65, No. 2, pp. 143–152.
Rights and permissions
About this article
Cite this article
Shibaeva, T.G., Sherudilo, E.G. & Titov, A.F. Response of Сucumber (Cucumis sativus L.) Plants to Prolonged Permanent and Short-Term Daily Exposures to Chilling Temperature. Russ J Plant Physiol 65, 286–294 (2018). https://doi.org/10.1134/S1021443718020061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443718020061