Abstract
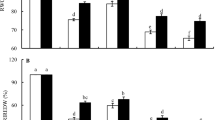
In order to explore the relationship between seed quality and changes in conjugated polyamine in plasma membrane purified from wheat embryos during grain ripening. Plasma membrane (PM) vesicles were isolated from the embryos of ripening wheat (Triticum aestivum L.) grains by the gradient centrifugation method. The contents of polyamines conjugated (covalently and non-covalently) to the PM vesicles were investigated. Results showed that after pollination, from the 22nd to the 32nd day, the embryos of wheat grains underwent dehydration, as judged by the decrease of embryo relative water content (ERWC). During embryo ripening, non-covalently conjugated (NCC)-Spd and NCC-Spm, covalently conjugated (CC)-Put and CC-Spd contents increased markedly, while relative embryo cell vigor (RECV) decreased slightly. The treatment with methylglyoxyl-bis (guanylhydrazone) (MGBG), an inhibitor of S-adenosylmethionine decarboxylase (SAMDC), inhibited the increases of the NCC-Spd and NCC-Spm contents, enhanced the decrease of RECV simultaneously, and decreased mature seed relative germination potential (RGP). The effects of MGBG were reversed by exogenous Spd and Spm. Phenanthrolin (o–Phen), an inhibitor of transglutaminase (TGase), inhibited the increases of CC-Put and CC-Spd contents, enhanced the decrease of RECV simultaneously, and decreased mature seed RGP. These results suggest that during embryo ripening, the levels of NCC-Spd, NCC-Spm, CC-Put, and CC-Spd increase, and these increases might affect embryo cell vigor and seed germination potential.
Similar content being viewed by others
Abbreviations
- CC:
-
covalently conjugated
- ERWC:
-
embryo relative water content
- MGBG:
-
methylglyoxyl-bis (guanylhydrazone)
- NCC:
-
noncovalently conjugated
- o–Phen:
-
phenanthrolin
- PA:
-
polyamine
- PCA:
-
perchloric acid
- PM:
-
plasma membrane
- Put:
-
putrescine
- PVP:
-
polyvinyl pyrrolidone
- RECV:
-
relative embryo cell vigor
- RGP:
-
relative germination potential
- SAMDC:
-
S-adenosylmethionine decarboxylase
- Spd:
-
spermidine
- Spm:
-
spermine
- TGase:
-
transglutaminase
- TTC:
-
triphenyltetrazolium chloride
References
Gupta, K., Dey, A., and Gupta, B., Plant polyamines in abiotic stress responses, Acta Physiol. Plant., 2013, vol. 35, pp. 2015–2036.
Slocum, R.D., Polyamine biosynthesis in plant, in Polyamines in Plants, Slocum, R.D. and Flores, H.E., Eds., Boca Raton, FL: CRC, 1991, pp. 23–40.
Yang, J.C., Cao, Y.Y., Zhang, H., Liu, L.J., and Zhang, J.H., Involvement of polyamines in the postanthesis development of inferior and superior spikelets in rice, Planta, 2008, vol. 228, pp. 137–149.
Chen, T.T., Xu, Y.J., Wang, J.C., Wang, Z.Q., Yang, J.C., and Zhang, J.H., Polyamines and ethylene interact in rice grains in response to soil drying during grain filling, J. Exp. Bot., 2013, vol. 64, pp. 2523–2538.
Legocka, J. and Kluk, A., Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus (L.) seedlings, J. Plant Physiol., 2005, vol. 162, pp. 662–668.
Galston, A.W. and Kaur-Sawhney, R., Polyamines as endogenous growth regulators, in Plant Hormones: Physiology, Biochemistry and Molecular Biology, Davies, P.J., Ed., Dordrecht: Kluwer, 1995, pp. 158–178.
Negrel, J., Javelle, F., and Paynot, M., Separation of putrescine and spermidine hydroxycinnamoyl transferases extracted from tobacco callus, Phytochemistry, 1991, vol. 30, pp. 1089–1092.
Mauricio, H.M., Jesus, N.R., and Catalina, P., Changes in polyamine content are related to low temperature resistance in potato plants, Acta Biol. Colomb., 1999, vol. 4, pp. 27–47.
Piqueras, A., Cortina, M.D., and Casas, J.L., Polyamines and hyperhydricity in micropropagated carnation plants, Plant Sci., 2002, vol. 162, pp. 671–678.
Kong, L.S., Attree, S.M., and Fowke, L.C., Effect of polyethylene glycol and methylglyoxal-bis (guanylhydrazone) on endogenous polyamine levels and somatic embryo maturation in white spruce, Plant Sci., 1998, vol. 133, pp. 211–220.
Scaramagli, S., Biondi, S., and Leone, A., Acclimation to low-water potential in potato cell suspension cultures leads to changes in putrescine metabolism, Plant Physiol. Biochem., 2000, vol. 38, pp. 345–351.
Liu, H.P., Dong, B.H., Zhang, Y.Y., Liu, Z.P., and Liu, Y.L., Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings, Plant Sci., 2004, vol. 166, pp. 1261–1267.
Chen, L., Liu, Q.Q., Gai, J.Y., Zhu, Y.L., Yang, L.F., and Wang, C., Effects of nitrogen forms on the growth and polyamine contents in developing seeds of vegetable soybean, J. Plant Nutr., 2011, vol. 34, pp. 504–521.
Wang, Z.Q., Xu, Y.J., Wang, J.C., Yang, J.C., and Zhang, J.H., Polyamine and ethylene interactions in grain filling of superior and inferior spikelets of rice, Plant Growth Regul., 2012, vol. 66, pp. 215–228.
Cao, D.D., Hu, J., Zhu, S.J., Hu, W.M., and Knapp, A., Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed, Sci. Hortic., 2010, vol. 123, pp. 301–307.
Yang, W.B., Yin, Y.P., Li, Y., Cai, T., Ni, Y.L., Peng, D.L., and Wang, Z.L., Interactions between polyamines and ethylene during grain filling in wheat grown under water deficit condition, Plant Growth Regul., 2014, vol. 72, pp. 189–201.
Shiozaki, S., Ogata, T., and Horiuchi, S., Endogenous polyamines in the pericarp and seed of the grape berry during development and ripening, Sci. Hortic., 2000, vol. 83, pp. 33–41.
Ching, T.M., Adenosine triphosphate content and seed vigour, Plant Physiol., 1973, vol. 51, pp. 400–402.
Kaur-Sawhney, R. and Shin, M., Relation of polyamines synthesized titer to aging and senescence in oat leaves, Plant Physiol., 1982, vol. 69, pp. 405–410.
Icekson, I. and Apelbaum, A., Evidence for transglutaminase activity in plant tissue, Plant Physiol., 1987, vol. 84, pp. 972–974.
Qiu, Q.S. and Su, X.F., The influence of extracellularside Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots, Aust. J. Plant Physiol., 1998, vol. 25, pp. 923–928.
Widell, S. and Larsson, C., A critical evaluation of markers used in plasma membrane purification, in The Plant Plasma Membrane, Larsson, C. and Moller, I.M., Eds., Berlin: Springer-Verlag, 1990, pp. 16–43.
Zhao, F.G., Sun, C., Liu, Y.L., and Liu, Z.P., Effect of salinity stress on the levels of covalently and noncovalently conjugated polyamines in plasma membrane and tonoplast isolated from barley seedlings, Acta Bot. Sinica, 2000, vol. 42, pp. 920–926.
Bradford, M.M., A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binging, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Sharma, P. and Rajam, M.V., Spatial and temporal changes in endogenous polyamine levels associated with osmotic embryogenesis from different hypocotyls segments of eggplant (Solanum melongena L.), J. Plant Physiol., 1995, vol. 146, pp. 658–664.
Di-Tomaso, J.M., Shaff, J.E., and Kochian, L.V., Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings, Plant Physiol., 1989, vol. 90, pp. 988–995.
Yamaguchi, K., Takahashi, Y., Berberich, T., Imai, A., Takahashi, T., Michael, A.J., and Kusano, T., A protective role for the polyamine spermine against drought stress in Arabidopsis, Biochem. Biophys. Res. Commun., 2007, vol. 352, pp. 486–490.
Farooq, M., Wahid, A., and Lee, D.J., Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties, Acta Physiol. Plant., 2009, vol. 31, pp. 937–945.
Radhakrishnan, R. and Lee, I.J., Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds, Acta Physiol. Plant., 2013, vol. 35, pp. 263–269.
Dutra, N.T., Silveira, V., Azevedo, I.G., Gomes-Neto, L.R., Facanha, A.R., Steiner, N., Guerra, M.P., Floh, E.I.S., and Santa-Catarina, C., Polyamines affect the cellular growth and structure of proembryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines, Physiol. Plant., 2013, vol. 148, pp. 121–132.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Du, H.Y., Zhou, X.G., Liu, G.T. et al. Relationship between seed quality and changes in conjugated polyamine in plasma membrane purified from wheat embryos during grain ripening. Russ J Plant Physiol 64, 325–332 (2017). https://doi.org/10.1134/S1021443717030074
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717030074