Abstract
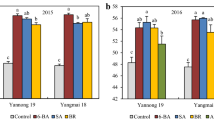
In order to determine the mechanism of laser-enhanced chilling tolerance in wheat (Triticum aestivum L.) seedlings, the seeds were exposed to different treatments, and some physiological and biochemical parameters were measured in 5-day-old seedlings. The results showed that in wheat seedlings subjected to 1-aminocyclopropane-1-carboxylic acid (ACC) followed by chilling stress (CS), the concentrations of H2O2, O2 .− and MDA were lower, while the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), as well as the soluble protein content (SPC) and the seedling length (SL) were higher than those in chilling stress treatment. When wheat seedlings were subjected to aminooxyacetic acid (AOA) followed by chilling stress, the results were different from those in which the seedlings were subjected to ACC. Although ACC and AOA treatments followed by laser irradiation led to an increase in the aforementioned enzyme activities, SPC, and SL, with a simultaneous decrease in concentrations of H2O2, O2 .−, and MDA, the effect of ACC treatment followed by laser irradiation was more favorable than that of AOA treatment followed by laser light. Similarly, the oxidative damage induced by chilling stress was alleviated in treatment with ACC+LR and AOA+LR, but alleviation effects of ACC+LR+CS were more prominent than in the treatment AOA+LR+CS. These results suggest that the laser light and ethylene have a positive synergistic effect, thus enhancing chilling tolerance in wheat seedlings.
Similar content being viewed by others
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- AOA:
-
aminooxyacetic acid
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- CS:
-
chilling stress
- GR:
-
glutathione reductase
- LR:
-
laser radiation
- POD:
-
peroxidases
- ROS:
-
reactive oxygen species
- SL:
-
shoot length
- SOD:
-
superoxide dismutase
- SPC:
-
soluble protein content
- ACC+CS:
-
treatment in which the seeds were incubated in the presence of ACC and then exposed to chilling stress
- ACC+LR:
-
seeds were treated with ACC followed by exposure to LR
- ACC+LR+CS:
-
seeds were treated with ACC followed by exposure to LR and CS
- AOA+CS:
-
seeds were treated with AOA followed by CS
- AOA+LR:
-
seeds were treated with AOA followed by exposure to LR
- AOA+LR+CS:
-
seeds were treated with AOA followed by exposure to LR and CS
References
Morsy, M.R., Jouve, L., Hausman, J.F., Hoffmann, L., and Stewart, J.M., Alteration of oxidative and carbohy-drate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance, J. Plant Physiol., 2007, vol. 164, pp. 157–167.
Wang, C.Y. and Adams, D.O., Chilling-induced ethylene production in cucumbers (Cucumis sativus L.), Plant Physiol., 1982, vol. 69, pp. 424–427.
Kacperska, A., Ethylene synthesis and a role in plant responses to different stressors, Biology and Biotechnology of the Plant Hormone Ethylene, Kanellis, A.K., Chang, C., Kende, H., Grierson, D., Eds., Dordrecht: Kluwer Academic, 1997, pp. 207–216.
Yang, S.F. and Hoffman, N.E., Ethylene biosynthesis and its regulation in higher plants, Annu. Rev. Plant Physiol., 1984, vol. 35, pp. 155–189.
Nandwal, A.S., Maan, A., Kundu, B.S., Sheokand, S., Kamboj, D.V., Sheoran, A., Kumar, B., and Dutta, D., Ethylene evolution and antioxidant defence mechanism in Cicer arietinum roots in the presence of nitrate and aminoethoxyvinylglycine, Plant Physiol. Biochem., 2000, vol. 38, pp. 709–715.
Larrigaudiere, C., Vilaplana, R., Soria, Y., and Recasens, I., Oxidative behaviour of Blanquilla pears treated with 1-methylcyclopropene during cold storage, J. Sci. Food Agric., 2004, vol. 84, pp. 1871–1877.
Lee, D.H. and Lee, C.B., Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays, Plant Sci., 2000, vol. 159, pp. 75–85.
Djanaguiraman, M., Sheeba, J.A., Devi, D.D., Bangarusamy, U., and Prasad, P.V.V., Nitrophenolates spray can alter boll abscission rate in cotton through enhanced peroxidase activity and increased ascorbate and phenolics levels, J. Plant Physiol., 2010, vol. 167, pp. 1–9.
Ramasarma, T., Many faces of superoxide dismutase, originally known as erythrocuprein, Curr. Sci., 2007, vol. 92, pp. 184–191.
Smirnoff, N., The role of active oxygen in the response of plants to water deficit and desiccation, New Phytol., 1993, vol. 125, pp. 27–58.
Corpas, F.J., Palma, J.M., Sandalio, L.M., Lopez-Huertas, E., Romero-Puertas, M.C., Barroso, J.B., and Del Río, L.A., Purification of catalase from pea leaf peroxisomes: identification of five different isoforms, Free Radic. Res., 1999, vol. 31, pp. 235–241.
Kawano, T., Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction, Plant Cell Rep., 2003, vol. 21, pp. 829–837.
Sakihama, Y., Cohen, M.F., Grace, S.C., and Yamasaki, H., Plant phenolic antioxidant and peroxidant activities: phenolics-induced oxidative damage mediated by metals in plants, Toxicology, 2002, vol. 177, pp. 67–80.
Perveen, R., Jamil, Y., Ashraf, M., Ali, Q., Iqbal, M., and Ahmad, M.R., He-Ne laser-induced improvement in biochemical, physiological, growth and yield characteristics in sunflower (Helianthus annuus L.), Photochem. Photobiol., 2011, vol. 87, pp. 1453–1463.
Qiu, Z.B., Liu, X., Tian, X.J., and Yue, M., Effects of CO2 laser pretreatment on drought stress resistance in wheat, Photochem. Photobiol., 2008, vol. 90, pp. 17–25.
Chen, Y.P., Jia, J.F., and Yue, M., Effect of CO2 laser radiation on physiological tolerance of wheat seedlings exposed to chilling stress, Photochem. Photobiol., 2010, vol. 86, pp. 600–605.
Predieri, S., Norman, H.A., Krizek, D.T., Pillai, P., Mirecki, R.M., and Zimmerman, R.H., Influence of UV-B radiation on membrane lipid composition and ethylene evolution in ‘Doyenne d’Hiver’ pear shoots grown in vitro under different photosynthetic photon fluxes, Environ. Exp. Bot., 1995, vol. 35, pp. 151–160.
Lin, Z.F., The accumulation of hydrogen peroxide in senescing leaves and chloroplasts in relation to lipid peroxidation, Acta Photophysiol. Sin., 1988, vol. 14, pp. 16–22.
Gao, J.F., Method of determining O2 .- in plant, Technology of Plant Physiological Experiment,Beijing: High Education Press, 2001.
Giannopolitis, C.N. and Ries, S.K., Superoxide dismutase. II. Purification and quantitative relationship with water-soluble protein in seedlings, Plant Physiol., 1977, vol. 59, pp. 315–318.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Cakmak, I. and Marschner, H., Magnesium deficiency and high light intensity enhance activity of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves, Plant Physiol., 1992, vol. 98, pp. 1222–1227.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biol. Chem., 1976, vol. 72, pp. 248–254.
Hoffman, N.E., Liu, Y., and Yang, S.F., Changes in 1-(malonylamino)-cyclopropane-L-carboxylic acid in wilted wheat leaves in relation to their ethylene production rates and L-aminocyclopropane-L-carboxylic acid content, Planta, 1983, vol. 157, pp. 518–523.
Chen, Y.Z. and Patte, B.D., Ethylene and L-aminocyclopropane-L-carboxylic acid as indicator of chilling sensitivity in various plant species, Aust. J. Plant Physiol., 1985, vol. 12, pp. 377–385.
Wang, H.H., Liang, X.L., Wan, Q., Wang, X.M., and Bi, Y.R., Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress, Planta, 2009, vol. 230, pp. 293–307.
Achard, P., Cheng, H., Grauwe, D.L., Decat, J., Schoutteten, H., Moritz, T., Straeten, D.V.D., Peng, J.R., and Harberd, N.P., Integration of plant responses to environmentally activated phytohormonal signals, Science, 2006, vol. 311, pp. 91–94.
Jung, J.Y., Shin, R., and Schachtman, D.P., Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis, Plant Cell, 2009, vol. 21, pp. 607–621.
Baker, A.J.M. and Walker, P.I., Physiological responses of plants to heavy metals and the quantification of tolerance and toxicity, Chem. Spec. Bioavailab., 1989, vol. 1, pp. 7–17.
Breckle, S.W. and Kahle, H., Effect of toxic heavy metals (Cd, ) on growth and mineral nutrition of beech (Fagus sylvatica L.), Vegetation, 1992, vol. 101, pp. 43–53.
Chen, Y.P., Liu, Y.J., Wang, X.L., Ren, Z.Y., and Yue, M., Effect of microwave and He-Ne laser on enzyme activity and biophoton emission of Isatis indigotica Fort, J. Integr. Plant Biol., 2005, vol. 47, pp. 849–855.
Liburdy, R.P. and Penn, A., Microwave bioeffects in erythrocytes are temperature and pO2 dependent: cation permeability and protein shedding occur at the membrane phase transition, Bioelectromagnetics, 1984, vol. 5, pp. 283–291.
Daniel, R.M., The upper limits of enzyme thermal stability, Enzyme Microb. Technol., 1996, vol. 19, pp. 74–79.
Yan, L.F. and Zhang, Y.L., Molecular Biology, Beijing: China Agricultural University Press, 1997 [in Chinese].
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Chen, Y.P., Liu, Q. Effect of laser irradiation and ethylene on chilling tolerance of wheat seedlings. Russ J Plant Physiol 62, 299–306 (2015). https://doi.org/10.1134/S1021443715030048
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443715030048