Abstract
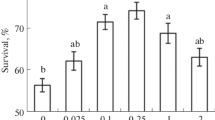
The involvement of nitric oxide (NO) and hydrogen peroxide (H2O2) in the formation of heat resistance induced by 1-min-long treatment with a temperature of 42°C in 3-day-old seedlings of winter soft wheat (Triticum aestivum L., cv. Elegiya) was studied. The content of NO in the roots was increased within 2 h after seedling hardening heating. The content of H2O2 was increased within 30 min after heating. This effect was neutralized when seedlings were treated with the nitric oxide scavenger PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) and the inhibitor of NO synthase L-NAME (NG-nitro-L-arginine methyl ester). Seedling treatment with the antioxidants ionol and dimethylthiourea (DMTU) reduced the hardening-induced nitric oxide accumulation in tissues. When seedlings were treated with the NO donor sodium nitroprusside (SNP), the amount of endogenous NO and H2O2 in them increased; exogenous hydrogen peroxide affected similarly. Hardening heating and treatment with SNP and hydrogen peroxide increased seedling resistance to damaging heating, whereas NO antagonists (PTIO and L-NAME) and antioxidants (ionol and DMTU) prevented the development of seedling heat resistance after hardening heating. It is concluded that, during the induction of wheat seedling heat resistance by the hardening heating, functional interaction between NO and H2O2 as signaling messengers occurs.
Similar content being viewed by others
Abbreviations
- DMTU:
-
dimethylthiourea
- L-NAME:
-
inhibitor of NO synthase (NG-nitro-L-arginine methyl ester)
- PTIO:
-
NO scavenger (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide)
- SNP:
-
sodium nitroprusside
References
Distefano, A.M., Lanteri, M.L., Have, A., Garcia-Mata, C., Lamattina, L., and Laxalt, A.M., Nitric oxide and phosphatidic acid signaling in plants, Lipid Signaling in Plants, Munnik, T., Ed., Berlin: SpringerVerlag, 2010, pp. 223–242.
Hasanuzzaman, M., Gill, S.S., and Fujita, M., Physiological role of nitric oxide in plants grown under adverse environmental conditions, Plant Acclimation to Environ-mental Stress, Tuteja, N. and Gill, S.S., Eds., New York: Springer Science+Business Media, 2013, pp. 269–322.
Prasad, T.K., Anderson, M.D., and Stewart, C.R., Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings, Plant Physiol., 1994, vol. 105, pp. 619–627.
Piotrovskii, M.S., Shevyreva, T.A., Zhestkova, I.M., and Trofimova, M.S., Activation of plasmalemmal NADPH oxidase in etiolated maize seedlings exposed to chilling temperatures, Russ. J. Plant Physiol., 2011, vol. 58, pp. 290–298.
Li, H.Y. and Li, C.G., Short-term cold-shock at 1 C induced chilling tolerance in maize seedlings, Proc. Int. Conf. Biology, Environment, Chemistry (IPCBEE), Singapore: IACSIT Press, 2011, vol. 1, pp. 346–349.
Kolupaev, Yu.Ye., Karpets, Yu.V., and Kosakivska, I.V., The importance of reactive oxygen species in the induction of plant resistance to heat stress, Gen. Appl. Plant Physiol., 2008, vol. 34, pp. 251–266.
Kolupaev, Yu.E., Oboznyi, A.I., and Shvidenko, N.V., Role of hydrogen peroxide in generation of a signal inducing heat tolerance of wheat seedlings, Russ. J. Plant Physiol., 2013, vol. 60, pp. 227–234.
Jiang, M. and Zhang, J., Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves, J. Exp. Bot., 2002, vol. 53, pp. 2401–2410.9.
Gould, K.S., Lamotte, O., Klinguer, A., Pugin, A., and Wendehenne, D., Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ., 2003, vol. 26, pp. 1851–1862.
Song, L., Ding, W., Zhao, M., Sun, B., and Zhang, L., Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed, Plant Sci., 2006, vol. 171, pp. 449–458.
Bakakina, Yu.S., Dubovskaya, L.V., and Volotovskii, I.D., Effect of high temperature stress on NO intracellular concentration and endogenous content of cGMP in Arabidopsis thaliana seedlings, Vestsi Nats. Akademii navuk Belarusi, Ser. Biol., 2009, no. 4, pp. 34–39.
Bakakina, Yu.S., Dubovskaya, L.V., and Volotovskii, I.D., Effect of cold stress on NO intracellular concentration and endogenous content of cGMP in Arabidopsis thaliana seedlings, Vestsi Nats. Akademii navuk Belarusi, Ser. Biol., 2009, no. 3, pp. 43–46.
Song, L., Zhao, H., and Hou, M., Involvement of nitric oxide in acquired thermotolerance of rice seedlings, Russ. J. Plant Physiol., 2013, vol. 60, pp. 785–790.
Wilson, I.D., Neill, S.J., and Hancock, J.T., Nitric oxide synthesis and signalling in plants, Plant Cell Environ., 2008, vol. 31, pp. 622–631.
Xu, M.J., Dong, J.F., and Zhang, X.B., Signal interaction between nitric oxide and hydrogen peroxide in heat shock-induced hypericin production of Hypericum perforatum suspension cells, Sci. China, Ser. C: Life Sci., 2008, vol. 51, pp. 676–686.
Tewari, R.K., Hahn, E.J., and Paek, K.Y., Function of nitric oxide and superoxide anion in the adventitious root development and antioxidant defence in Panax ginseng, Plant Cell Rep., 2008, vol. 27, pp. 563–573.
Karpets, Yu.V., Kolupaev, Yu.E., and Yastreb, T.O., Effect of sodium nitroprusside on heat resistance of wheat coleoptiles: dependence on the formation and scavenging of reactive oxygen species, Russ. J. Plant Physiol., 2011, vol. 58, pp. 1027–1034.
Lu, D., Zhang, X., Jiang, J., An, G.Y., Zhang, L.R., and Song, C.P., NO may function in the downstream of H2O2 in ABA-induced stomatal closure in Vicia faba L., J. Plant Physiol. Mol. Biol., 2005, vol. 31, pp. 62–70.
Zhang, A., Jiang, M., Zhang, J., Ding, H., Xu, S., Hu, X., and Tan, M., Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves, New Phytol., 2007, vol. 175, pp. 36–50.
Akaike, T., Yoshida, M., Miyamoto, Y., Sato, K., Kohno, M., Sasamoto, K., Miyazaki, K., Ueda, S., and Maeda, H., Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/NO through a radical reaction, Biochemistry, 1993, vol. 32, pp. 827–832.
Zhou, B., Guo, Z., Xing, J., and Huang, B., Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis, J. Exp. Bot., 2005, vol. 56, pp. 3223–3228.
Sagisaka, S., The occurrence of peroxide in a perennial plant, Populus gelrica, Plant Physiol., 1976, vol. 57, pp. 308–309.
Tanou, G., Job, C., Belghazi, M., Molassiotis, A., Diamantidis, G., and Job, D., Proteomic signatures uncover hydrogen peroxide and nitric oxide cross-talk signaling network in citrus plants, J. Proteome Res., 2010, vol. 9, pp. 5994–6006.
Vital, S.A., Fowler, R.W., Virgen, A., Gossett, D.R., Banks, S.W., and Rodriguez, J., Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue, Environ. Exp. Bot., 2008, vol. 62, pp. 60–68.
Reiter, C.D., Teng, R.J., and Beckman, J.S., Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxinitrite, J. Biol. Chem., 2000, vol. 275, pp. 32 460–32 466.
Dubovskaya, L.V., Kolesneva, E.V., Knyazev, D.M., and Volotovskii, I.D., Protective role of nitric oxide during hydrogen peroxide-induced oxidative stress in tobacco plants, Russ. J. Plant Physiol., 2007, vol. 54, pp. 755–762.
Neill, S., Bright, J., Desikan, R., Hancock, J., Harrison, J., and Wilson, I., Nitric oxide evolution and perception, J. Exp. Bot., 2008, vol. 59, pp. 25–35.
Glyan’ko, A.K. and Ishchenko, A.A., Structural and functional characteristics of plant NADPH oxidase: A review, Appl. Biochem. Microbiol., 2010, vol. 46, pp. 463–471.
Liu, H.T., Li, B., Shang, Z.L., Li, X.Z., Mu, R.L., Sun, D.Y., and Zhou, R.G., Calmodulin is involved in heat shock signal transduction in wheat, Plant Physiol., 2003, vol. 132, pp. 1186–1195.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.V. Karpets, Yu.E. Kolupaev, A.A. Vayner, 2015, published in Fiziologiya Rastenii, 2015, Vol. 62, No. 1, pp. 72–78.
Rights and permissions
About this article
Cite this article
Karpets, Y.V., Kolupaev, Y.E. & Vayner, A.A. Functional interaction between nitric oxide and hydrogen peroxide during formation of wheat seedling induced heat resistance. Russ J Plant Physiol 62, 65–70 (2015). https://doi.org/10.1134/S1021443714060090
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443714060090