Abstract
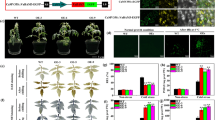
Drought is the major abiotic stress restricting plant growth. To gain insight into the regulatory mechanisms for drought stress tolerance, we performed a genetic screen for identifying Arabidopsis (Arabidopsis thaliana) drought-resistant mutants. A drought-resistant mutant vrm1 was isolated because of its increased root growth and fresh weight under drought stress. Compared with the wild type (WT), the vrm1 mutant was more resistant to drought stress. The vrm1 plants showed the higher levels of free proline and soluble sugars and the lower relative stomatal conductance as well as the lower water loss rate than the WT under drought stress. Moreover, the higher transcription levels of drought stress-responsive genes NCED3, RD22, RD29A, and COR15A were detected in vrm1 plants subjected to drought and mannitol stresses as compared with WT plants. These results suggest that increased drought resistance of the vrm1 mutant was associated with, at least in part, the higher expression of these drought stress-responsive genes.
Similar content being viewed by others
Abbreviations
- Col-0:
-
Colombia-0
- EMS:
-
ethyl methane sulfonate
- vrm1 :
-
drought-resistant mutant
- WT:
-
wild type
References
Neumann, P.M., Coping mechanisms for crop plants in drought-prone environments, Ann. Bot., 2008, vol. 101, pp. 901–907.
Comstock, J.P., Hydraulic and chemical signaling in the control of stomatal conductance and transpiration, J. Exp. Bot., 2002, vol. 53, pp. 195–200.
Harb, A., Krishnan, A., Amavaram, M.M.R., and Pereira, A., Molecular and physiological analysis of drought stress in Arabidopsis reveals early response leading to acclimation in plant growth, Plant Physiol., 2010, vol. 154, pp. 1254–1271.
Qin, F., Sakuma, Y., Tran, L.S.P., Maruyama, K., Kidokoro, S., Fujita, Y., Fujita, M., Umezawa, T., Sawano, Y., Miyazono, K.I., Tanokura, M., Shinozaki, K., and Yamaguchi-Shinozaki, M., Arabidopsis DREB2A-interacting proteins function as ring E3 ligases and negatively regulate plant drought stress-responsive gene expression, Plant Cell, 2008, vol. 20, pp. 1693–1707.
Alexandersson, E., Danielson, J.A.H., Rade, J., Moparthi, V.K., Fontes, M., Kjellbom, P., and Johanson, U., Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress, Plant J., 2010, vol. 61, pp. 650–660.
Yoshida, T., Fujita, Y., Sayama, H., Kyonoshin, S., Maruyama, K., Mizoi, J., Shinozaki, K., and Shinozaki, K.Y., AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation, Plant J., 2010, vol. 61, pp. 672–685.
Wang, Z.Y., Xiong, L.M., Li, W.B., Zhu, J.K., and Zhu, J.H., The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis, Plant Cell, 2011, vol. 23, pp. 1971–1984.
Yu, H., Chen, X., Hong, Y.Y., Wang, Y., Xu, P., Ke, S.D., Liu, H.Y., Zhu, J.K., Oliver, D.J., and Xiang, C.B., Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density, Plant Cell, 2008, vol. 20, pp. 1134–1151.
Chen, J.H., Jiang, H.W., Hsieh, E.J., Chen, H.Y., Chien, C.T., Hsieh, H.L., and Lin, T.P., Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid, Plant Physiol., 2012, vol. 158, pp. 340–351.
Jiang, L., Yang, R.Z., Lu, Y.F., Cao, S.Q., Ci, L.K., and Zhang, J.J., β-Aminobutyric acid-mediated tobacco tolerance to potassium deficiency, Russ. J. Plant Physiol., 2012, vol. 59, pp. 781–787.
Chen, A.K., Han, R.H., Li, D.Y., Ling, L.L., Luo, H.X., and Tang, S.J., The study on comparison of different relative conductance measurements for plant leaves, J. Guangdong Education Inst., 2010, vol. 30, pp. 88–91.
Smart, R.E. and Bingham, G.E., Rapid estimates of relative water content, Plant Physiol., 1974, vol. 53, pp. 258–260.
Yuan, J.S., Reed, A., Chen, F., and Stewart, C.N., Statistical analysis of real-time PCR data, BMC Bioinformatics, 2006, vol. 7, p. 85.
Zhao, J.S., Ren, W., Zhi, D.Y., Wang, L., and Xia, G.M., Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress, Plant Cell Rep., 2007, vol. 26, pp. 1521–1528.
Kantar, M., Lucas, S.J., and Budak, H., miRNA expression patterns of Triticum dicoccoides in response to shock drought stress, Planta, 2011, vol. 233, pp. 471–484.
Uno, Y., Furihata, T., Abe, H., Yoshida, R., Shinozaki, K., and Yamaguchi-Shinozaki, K., Arabidopsis basic leucine zipper transcriptional transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions, Proc. Natl. Acad. Sci. USA, 2000, vol. 97, pp. 11 632–11 637.
Shinozaki, K. and Yamaguchi-Shinozaki, K., Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways, Curr. Opin. Plant Biol., 2000, vol. 3, pp. 217–223.
Shinozaki, K., Yamaguchi-Shinozaki, K., and Seki, M., Regulatory network of gene expression in the drought and cold stress responses, Curr. Opin. Plant Biol., 2003, vol. 6, pp. 410–417.
Zou, J.J., Wei, F.J., Wang, C., Wu, J.J., Ratnasekera, D., Liu, W.X., and Wu, W.H., Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress, Plant Physiol., 2010, vol. 154, pp. 1232–1243.
Lee, S.J., Jung, H.J., Kang, H.S., and Kim, S.Y., Arabidopsis zinc finger proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are involved in ABA and JA responses, Plant Cell Physiol., 2012, vol. 53, pp. 673–686.
Author information
Authors and Affiliations
Corresponding author
Additional information
This text is submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Jiang, L., Chen, Z.P., Zhang, J.J. et al. Isolation and characterization of an arabidopsis drought-resistant mutant vrm1 . Russ J Plant Physiol 60, 830–838 (2013). https://doi.org/10.1134/S1021443713060046
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443713060046