Abstract
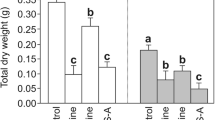
Seven-day-old kidney bean and cabbage seedlings were treated with 0.1–0.3 M NaCl solutions for 3 days. Chlorophyll content decreased in NaCl-treated Phaseolus seedlings, but did not significantly decrease in Brassica seedlings. Photochemical efficiency of photosystem II at dark-adapted state was similar in both Phaseolus and Brassica. The de-epoxidation state of violaxanthin increased more than sixfold in Phaseolus but showed no significant change in Brassica seedlings during NaCl treatment under low light. Maximum de-epoxidation state of violaxanthin in vivo tested in high light (2000 μmol quanta/(m2 s) increased in salt-stressed Phaseolus but decreased in Brassica seedlings. The nonphotochemical quenching (NPQ) also increased in Phaseolus but decreased in Brassica. This suggests that xanthophyll cycle pigments influence the NPQ in both Phaseolus and Brassica, but in an opposite way. The increase in the de-epoxidation state of violaxanthin in salt-stressed Phaseolus even under low light may be considered an early light signal to protect the pigment-protein complexes from salt-stress induced photodamage. It is proposed that in salt-stressed Brassica, the de-epoxidation is retarded and/or the epoxidation is accelerated leading to the accumulation of violaxanthin and a lower de-epoxidation state. Thus, light-induced violoxanthin cycle operation largely controls the photoprotection of photosynthetic apparatus in kidney bean leaves.
Similar content being viewed by others
Abbreviations
- A:
-
antheraxanthin
- Car:
-
carotenes
- Chl a and b :
-
chlorophylls a and b
- F v /F m :
-
photochemical efficiency of PSII at dark adapted state
- LHC:
-
light-harvesting antenna complex
- L:
-
lutein
- N:
-
neoxanthin
- NPQ:
-
nonphotochemical quenching
- PAR:
-
photosynthetically active radiation
- PS:
-
photosystem
- PQ:
-
plastoquinone
- V:
-
violaxanthin
- VDE:
-
violaxanthin de-epoxidase
- Z:
-
zeaxanthin
References
Flowers, T.J. and Yeo, A.R., Breeding for Salinity Tolerance in Crop Plants. Where Next? Aust. J. Plant Physiol., 1995, vol. 22, pp. 875–884.
Apse, M.P. and Blumwald, E., Engineering Salt Tolerance in Plants, Curr. Opin. Biotechnol., 2002, vol. 13, pp. 146–150.
Tester, M. and Davenport, R., Na+ Tolerance and Na+ Transport in Higher Plants, Ann. Bot., 2003, vol. 91, pp. 503–527.
Misra, A.N., Sahu, S.M., Misra, M., Mohapatra, P., Meera, I., and Das, N., Root Growth of a Salt Susceptible and a Salt Resistant Rice (Oryza sativa L.) during Seedling Establishment under NaCl Salinity, J. Agron. Crop Sci., 1997, vol. 178, pp. 9–14.
Misra, A.N., Sahu, S.M., Misra, M., Singh, P., Meera, I., Das, N., Kar, M., and Sahu, P., Sodium Chloride Induced Changes in Leaf Growth, and Pigment and Protein Contents in Two Rice Cultivars, Biol. Plant., 1997, vol. 39, pp. 257–262.
Misra, A.N., Sahu, S.M., Misra, M., Ramaswamy, N.K., and Desai, T.S., Sodium Chloride Salt Stress Induced Changes in Thylakoid Pigment-Protein Complexes, Photosystem II Activity and Thermoluminescence Glow Peaks, Z. Naturforsch, 1999, vol. 54, pp. 640–644.
Demmig-Adams, B. and Adams, W.W., Antioxidants in Photosynthesis and Human Nutrition, Science, 2002, vol. 298, pp. 2149–2153.
Misra, A.N., Srivastava, A., and Strasser, R.J., Utilization of Fast Chlorophyll a Fluorescence Technique in Assessing the Salt/Ion Sensitivity of Mung Bean and Brassica Seedlings, J. Plant Physiol., 2001, vol. 158, pp. 1173–1181.
Sahu, S.M., Misra, A.N., Misra, M., Ramaswamy, N.K., and Desai, T.S., Sodium Chloride Salt Stress Induced Changes in Thylakoid Pigment-Protein Complexes, PS II Activity of Mungbean (Vigna radiate L.) Seedlings, Photosynthesis: Mechanism and Effects, Garab, G., Ed., Dordrecht: Kluwer, 1998, pp. 2625–2628.
Muller, P., Li, X.-P., and Niyogi, K.K., Non-Photochemical Quenching. A Response to Excess Light Energy, Plant Physiol., 2001, vol. 125, pp. 1558–1566.
Pogson, B.J., Niyogi, K.K., Bjorkman, O., and Della Penna, D., Altered Xanthophylls Compositions Adversely Affect Chlorophyll Accumulation and Non-Photochemical Quenching in Arabidopsis Mutants, Proc. Natl. Acad. Sci. USA, 1998, vol. 95, pp. 13324–13329.
Latowski, D., Grzyb, J., and Strzalka, K., The Xanthophyll Cycle — Molecular Mechanism and Physiological Significance, Acta Physiol. Plant., 2004, vol. 26, pp. 197–212.
Kuhlbrandt, W., Wang, D.N., and Fujiyoshi, Y., Atomic Model of Plant Light Harvesting Complex by Electron Crystallography, Nature, 1994, vol. 367, pp. 614–621.
Johnson, G.N., Scholes, J.D., Horton, P., and Young, A.J., Relationship between Carotenoid Composition and Growth Habit in British Plant Species, Plant, Cell Environ., 1993, vol. 16, pp. 681–686.
Neubauer, C. and Yamamoto, H.E., Membrane Barriers and Mehler Peroxidase Reaction Limit the Ascorbate Availability for Violaxanthin De-Epoxidase Activity in Intact Chloroplasts, Photosynth. Res., 1994, vol. 39, pp. 137–147.
Sieferman, D. and Yamamoto, H.E., Properties of NADPH and Oxygen Dependent Zeaxanthin Epoxidation of Isolated Chloroplasts, Arch. Biochem. Biophys., 1962, vol. 171, pp. 70–77.
Davison, P.A., Hunter, C.N., and Horton, P., Overexpression of β-Carotene Hydroxylase Enhances Stress Tolerance in Arabidopsis, Nature, 2002, vol. 418, pp. 203–206.
Asada, K., The Water-Water Cycle in Chloroplasts: Scavenging of Active Oxygen and Dissipation of Excess Photons, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1999, vol. 50, pp. 601–639.
Foyer, C.H. and Noctor, G., Oxygen Processing in Photosynthesis: Regulation and Signaling, New Phytol., 2000, vol. 146, pp. 359–388.
Jezowska, I., Wolak, A., Gruszecki, W.I., and Strzalka, K., Effect of β-Carotene on Structural and Dynamic Properties of Model Phosphatidylcholine Membranes: 2. A 31P-NMR and 13C-NMR Study, Biochim. Biophys. Acta, 1994, vol. 1194, pp. 143–148.
Strzalka, K. and Gruszecki, W.I., Effect of β-Carotene on Structural and Dynamic Properties of Model Phosphatidylcholine Membranes: 1. An EPR Spin Label Study, Biochim. Biophys. Acta, 1994, vol. 1194, pp. 138–142.
Gruszecki, W.I. and Strzalka, K., Carotenoids as Modulators of Lipid Membrane Physical Properties, Biochim. Biophys. Acta, 2005, vol. 1740, pp. 108–115.
Kostecka-Gugala, A., Latowski, D., and Strzalka, K., Thermotropic Phase Behaviour of α-Dipalmitoylphosphatidylcholine Multibilayers Is Influenced to Various Extents by Carotenoids Containing Different Structural Features — Evidence from Differential Scanning Calorimetry, Biochim. Biophys. Acta, 2003, vol. 1609, pp. 193–202.
Gruszecki, W.I. and Strzalka, K., Does the Xanthophyll Cycle Take Part in the Regulation of Fluidity of the Thylakoid Membrane? Biochim. Biophys. Acta, 1991, vol. 1060, pp. 310–314.
Latowski, D., Kruk, J., Burda, K., Skrzynecka-Jaskier, M., Kostecka-Gugala, A., and Strzalka, K., Kinetics of Violaxanthin De-Epoxidation by Violaxanthin De-Epoxidase, a Xanthophyll Cycle Enzyme, Is Regulated by Membrane Fluidity in Model Lipid Bilayers, Eur. J. Biochem., 2002, vol. 269, pp. 4656–4665.
Latowski, D., Akerlund, H.-E., and Strzalka, K., Violaxanthin De-Epoxidase, the Xanthophyll Cycle Enzyme, Requires Lipid Inverted Hexagonal Structures for Its Activity, Biochemistry, 2004, vol. 43, pp. 4417–4420.
Subczynski, W.K., Markowska, E., and Sielewiesiuk, J., Effect of Polar Carotenoids on the Oxygen Diffusion-Concentration Product in Lipid Bilayers, Biochim. Biophys. Acta, 1991, vol. 1068, pp. 68–72.
Strzalka, K. and Gruszecki, W.I., Modulation of Thylakoid Membrane Fluidity by Exogenously Added Carotenoids, J. Biochem. Mol. Biol. Biophys., 1997, vol. 1, pp. 103–108.
Tardy, F. and Havaux, M., Photosynthesis, Chlorophyll Fluorescence, Light Harvesting System and Photoinhibition Resistance of a Zeaxanthin-Accumulating Mutant of Arabidopsis thaliana, J. Photochem. Photobiol. Ser. B., 1996, vol. 34, pp. 87–94.
Hirschberg, J., Carotenoid Biosynthesis in Flowering Plants, Curr. Opin. Plant Biol., 2001, vol. 4, pp. 210–218.
Milborrow, B.V. and Lee, H.S., Endogenous Biosynthetic Precursor of (+)-Abscisic Acid. VI. Carotenoids and ABA Are Formed by the ‘Non-Mevalonate’ Triosepyruvate Pathway in Chloroplasts, Aust. J. Plant Physiol., 1998, vol. 25, pp. 507–512.
Cramer, G.R. and Quarrie, S.A., Abscisic Acid Is Correlated with Leaf Growth Inhibition of Four Genotypes of Maize Differing in Their Response to Salinity, Funct. Plant Biol., 2002, vol. 29, pp. 111–119.
Makela, P., Munns, R., Colmer, T.D., and Pelton-Sainio, P., Growth of Tomato and an ABA-Deficient Mutant (sitiens) under Saline Conditions, Physiol. Plant., 2003, vol. 17, pp. 58–63.
Arnon, D.I., Copper Enzyme in Isolated Chloroplasts. Polyphenol Oxidase in Beta vulgaris, Plant Physiol., 1949, vol. 24, pp. 1–15.
Gilmore, A.M. and Yamamoto, H.Y., Resolution of Lutein and Zeaxanthin Using a Non-Endcapped, Lightly Carbon Loaded C18 High-Performance Liquid Chromatographic Column, J. Chromatogr., 1991, vol. 35, pp. 67–78.
Farber, A., Young, A.J., Ruban, A.V., Horton, P., and Jahns, P., Dynamics of Xanthophylls Cycle Activity in Different Antenna Subcomplexes in Photosynthetic Membranes of Higher Plants. The Relationship between Zeaxanthin Conversion and Non-Photochemical Fluorescence Quenching, Plant Physiol., 1997, vol. 115, pp. 1609–1618.
Demmig, B., Winter, K., Kruger, A., and Czygan, F.-C., Photoinhibition and Zeaxanthin Formation in Intact Leaves, Plant Physiol., 1987, vol. 84, pp. 218–224.
Thayer, S.S. and Bjorkman, O., Leaf Xanthophyll Content and Composition in Sun and Shade Determined by HPLC, Photosynth. Res., 1990, vol. 23, pp. 331–343.
Biswal, A.K., Dilnawaz, F., Ramaswamy, N.K., David, K.A.V., and Misra, A.N., Thermoluminescence Characteristics of Sodium Chloride Salt Stressed Indian Mustard Seedlings, Luminescence, 2002, vol. 17, pp. 135–140.
Misra, A.N., Sahu, S.M., and Misra, M., Soil Salinity Induced Changes in Pigment and Protein Contents in Cotyledons and Leaves of Indian Mustard (Brassica juncea Coss.), Acta Physiol. Plant., 1995, vol. 17, pp. 375–380.
Horton, P., Ruban, A.V., and Walters, R.G., Regulation of Light Harvesting in Green Plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1996, vol. 47, pp. 655–684.
Havaux, M., Bonfils, J., Lutz, C., and Niyogi, K., Photodamage of the Photosynthetic Apparatus and Its Dependence on the Leaf Developmental Stage in the nq1 Arabidopsis Mutant Deficient in the Xanthophyll Cycle Enzyme Violaxanthin De-Epoxidase, Plant Physiol., 2000, vol. 124, pp. 273–284.
Hurry, V., Anderson, J.M., Chow, W.S., and Osmond, C.B., Accumulation of Zeaxanthin in Abscisic Acid Deficient Mutant of Arabidopsis Does Not Affect Chlorophyll Fluorescence Quenching or Photosensitivity to Photoinhibition In Vivo, Plant Physiol., 1997, vol. 116, pp. 173–181.
Niyogi, K.K., Bjorkman, O., and Grossman, A.R., The Role of Specific Xanthophylls in Photoprotection, Proc. Natl. Acad. Sci. USA, 1997, vol. 94, pp. 14 162–14 167.
Yamamoto, H.E., Nakayama, T.O.M., and Chichester, C.O., Studies on the Light and Dark Interconverions of Xanthophylls, Arch. Biochem. Biophys., 1962, vol. 97, pp. 168–173.
Bratt, C.E., Arvidsson, P.-O., Carlsson, M., and Akerlund, H.-E., Regulation of Violaxanthin De-Epoxidase Activity by pH and Ascorbate Concentration, Photosynth. Res., 1995, vol. 45, pp. 169–175.
Author information
Authors and Affiliations
Additional information
Published in Russian in Fiziologiya Rastenii, 2006, Vol. 53, No. 1, pp. 113–121.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Misra, A.N., Latowski, D. & Strzalka, K. The xanthophyll cycle activity in kidney bean and cabbage leaves under salinity stress. Russ J Plant Physiol 53, 102–109 (2006). https://doi.org/10.1134/S1021443706010134
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1021443706010134