Abstract
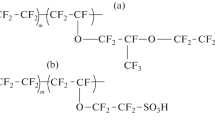
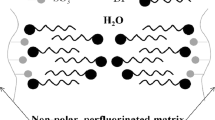
The thermodynamics of interaction between poly(perfluorosulfonic acid) Nafion and water is studied by isothermal sorption and microcalorimetry. The concentration dependences of energy and entropy parameters of mixing of Nafion aqueous solutions are determined. It is shown that the Gibbs energy and the enthalpy of mixing are negative while the entropy of mixing is positive over the entire range of solution compositions. The experimental water sorption isotherms and the concentration dependences of the enthalpy of dilution of aqueous solutions are analyzed in terms of the thermodynamic model allowing for pair nonvalence interactions in solution, nonequilibrium glassy structure of the polymer, and effects of the dissociation of ionic sulfo groups of Nafion. The calculated value of the Flory–Huggins parameter is 1.48, and the value of its enthalpy component is close to zero.





Similar content being viewed by others
REFERENCES
A. Kusoglu and A. Z. Weber, Chem. Rev. 117 (3), 987 (2017).
J. Kim, K. Yamasaki, H. Ishimoto, and Y. Takata, Polymers 1 (3), 1730 (2020).
L. Mazzapioda, VecchioC. Lo, O. Danyliv, V. Baglio, A. Martinelli, and M. A. Navarra, Polymers 12 (3), 2019 (2020).
H. G. Haubold, T. Vad, H. Jungbluth, and P. Hiller, Electrochim. Acta 46 (10), 1559–1563 (2001).
Perfluorinated Ionomer Membranes, Ed. by H. L. Yeager and A. Eisenberg, ACS Symposium Series (American Chemical Society, Washington DC, 1982), Vol. 180, Chap. 4, p. 41.
K. Schmidt-Rohr and Q. Chen, Nature Mater. 7, 75 (2008).
N. A. Ivanova, D. D. Spasov, S. A. Grigoriev, and V. N. Fateev, Polymers 14 (20), 4395 (2022).
T. Thampan, S. Malhotra, H. Tang, and R. Datta, J. Electrochem. Soc. 147 (9), 3242 (2000).
D. R. Morris and X. Sun, J. Appl. Polym. Sci. 50 (8), 1445 (1993).
M. Pineri, F. Volino, and M. Escoubes, J. Polym. Sci., Polym. Phys. Ed. 23 (10), 2009 (1985).
T. A. Zawodzinski, T. E. Springer, J. Davey, R. Jestel, C. Lopez, J. Valerio, and S. Gottesfeld, J. Electrochem. Soc. 140 (7), 1981 (1993).
M. Laporta, M. Pegoraro, and L. Zanderighi, Phys. Chem. Chem. Phys. 1 (19), 4619 (1999).
T. A. Zawodzinski, C. Derouin, S. Radzinski, R. J. Sherman, V. T. Smith, T. E. Springer, and S. Gottesfeld, J. Electrochem. Soc. 140 (4), 1041 (1993).
P. J. James, J. A. Elliott, T. J. McMaster, J. M. Newton, A. M. Elliott, S. Hanna, and M. J. Miles, J. Mater. Sci. 35 (20), 5111 (2000).
J. T. Hinatsu, M. Mizuhata, and H. Takenaka, J. Electrochem. Soc. 141 (6), 1493 (1994).
C. Vallieres, D. Winkelmann, D. Roizard, E. Favre, P. Scharfer, and M. Kind, J. Membr. Sci. 278 (1–2), 357 (2006).
P. Choi and R. Datta, ACS Div. Fuel Chem. Prepr. 48 (1), 300 (2003).
A. Z. Weber and J. Newman, J. Electrochem. Soc. A 151 (2), 311 (2004).
P. J. Reucroft, D. Rivin, and N. S. Schneider, Polymer 43 (19), 5157 (2002).
R. L. Benoit and D. Figeys, Can. J. Chem. 69 (12), 1985 (1991).
M. Noppel, J. Geophys. Res.: Atmospheres 105 (15), 19779 (2000).
D. M. T. Newsham and E. J. Mendez-Lecanda, J. Chem. Thermodyn. 14 (3), 291 (1982).
V. E. Ostrovskii and B. V. Gostev, J. Therm. Anal. 46 (2), 397 (1996).
A. Kusoglu, S. Savagatrup, K. T. Clark, and A. Z. Weber, Macromolecules 45 (18), 7467 (2012).
M. H. Kim, C. J. Glinka, S. A. Grot, and W. G. Grot, Macromolecules 39 (14), 4775 (2006).
S. W. Shi, T. J. Dursch, C. Blake, R. Mukundan, R. L. Borup, A. Z. Weber, and A. Kusoglu, J. Polym. Sci., Polym. Phys. Ed. 54 (5), 570 (2016).
J. S. Li, X. Yang, H. L. Tang, and M. Pan, J. Membr. Sci. 361 (1–2), 38 (2010).
A. P. Safronov and L. V. Adamova, Polym. Sci., Ser. A 44 (4), 408 (2002).
A. P. Safronov and T. V. Terziyan, Polym. Sci., Ser. A 50 (7), 733 (2008).
R. S. Yeo, Polymer 21 (4), 432 (1980).
T. H. Mourey, L. A. Slater, R. C. Galipo, and R. J. Koestner, J. Chromatogr., A 1218 (34), 5801 (2011).
A. E. Chalykh, V. K. Gerasimov, and V. G. Chertkov, Vysokomol. Soedin., Ser. B 36 (12), 2077 (1994).
A. A. Tager, Physical Chemistry of Polymers (Ripol Klassik, Moscow, 1978) [in Russian].
A. P. Safronov, L. V. Adamova, A. S. Blokhina, I. A. Kamalov, and P. A. Shabadrov, Polym. Sci., Ser. A 57 (1), 33 (2015).
D. Chu, D. Tryk, D. Gervasio, and E. B. Yeager, J. Electroanal. Chem. 272 (1–2), 277 (1989).
P. Choi, N. H. Jalani, and R. Datta, J. Electrochem. Soc. 152 (3), E123 (2005).
D. Wang and C. J. Cornelius, J. Polym. Sci., Polym. Phys. Ed. 55 (5), 435 (2017).
K. Shinoda, J. Phys. Chem. 81 (13), 1300 (1977).
ACKNOWLEDGMENTS
We are grateful to E.D. Kuznetsova for her help in sorption measurements.
Funding
This work was carried out in accordance with the State Assignment for the Institute of Solid State Chemistry, Urals Branch, Russian Academy of Sciences, theme no. 0320-2019-0005 (Registration number NIOKTR АААА-А19-119102990044-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Soboleva
Rights and permissions
About this article
Cite this article
Chernyuk, S.D., Safronov, A.P., Adamova, L.V. et al. Thermodynamics of Interaction between Poly(perfluorosulfonic acid) Nafion and Water. Polym. Sci. Ser. A 65, 119–127 (2023). https://doi.org/10.1134/S0965545X23700839
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X23700839