Abstract
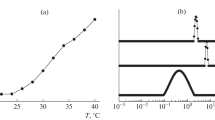
Random and protein-like copolymers based on N-vinylcaprolactam and N-vinylimidazole are synthesized by free radical polymerization in an aqueous solution. The above copolymers show a different thermal behavior in aqueous media at pH 7.2. At 45°C, the solution of a random copolymer experiences phase separation, whereas a protein-like copolymer undergoes a transition from the unfolded conformation to the compact conformation without any phase separation. The method of isothermal titration calorimetry is used to study the binding of Cu(II) ions by protein-like and random copolymers of N-vinylcaprolactam and N-vinylimidazole at 25 and 45°C, which correspond to different conformational states of macromolecules. The standard enthalpy and constant of binding are estimated. For both copolymers, the enthalpies of binding are negative and similar. When temperature is increased from 25 to 45°C, the constant of binding of copper ions by a protein-like copolymer increases by more than three orders of magnitude, whereas the corresponding constant of a random copolymer remains almost unchanged. Therefore, the transition of protein-like copolymer from the coiled conformation to the compact conformation noticeably facilitates the formation of an imidazole quasi-receptor, which is characterized by a certain spatial configuration and by a high affinity for the functional ligand. This effect is provided by an entropy gain no less than 50 J/(mol K).
Similar content being viewed by others
References
A. L. Lehninger, Principles of Biochemistry (Worth, New York, 1982).
A. V. Finkel’shtein and O. B. Ptitsyn, Physics of Proteins (Knizhnyi Dom “Universitet”, Moscow, 2002) [in Russian].
A. R. Khokhlov and P. G. Khalatur, Curr. Opin. Solid State Mater. Sci. 8, 3 (2004).
R. Breslow, J. Biol. Chem. 284, 1337 (2009).
P. G. Khalatur and A. R. Khokhlov, Adv. Polym. Sci. 195, 1 (2006).
A. N. Semenov, Macromolecules 37, 226 (2004).
A. R. Khokhlov and P. G. Khalatur, Physica A (Amsterdam) 249, 253 (1998).
A. R. Khokhlov and P. G. Khalatur, Phys. Rev. Lett. 82, 3456 (1999).
A. R. Khokhlov, A. V. Berezkin, and P. G. Khalatur, J. Polym. Sci., Part A: Polym. Chem. 42, 5339 (2004).
N. M. Bergmann and N. A. Peppas, Prog. Polym. Sci. 33, 271 (2008).
V. I. Lozinsky, Adv. Polym. Sci. 196, 87 (2006).
M. Siu, G. Zhang, and C. Wu, Macromolecules 35, 2723 (2002).
V. I. Lozinsky, I. A. Simenel, V. K. Kulakova, et al., Macromolecules 36, 7308 (2003).
V. I. Lozinskii, I. A. Simenel, E. A. Kurskaya, et al., Dokl. Akad. Nauk 375, 637 (2000).
V. I. Lozinskii, I. A. Simenel, M. G. Semenova, et al., Polymer Science, Ser. A 48, 435 (2006) [Vysokomol. Soedin., Ser. A 48, 673 (2006)].
D. H. Gold and H. P. Gregor, J. Phys. Chem., A 64, 1464 (1960).
Y. Zhang, S. Akilesh, and D. E. Wilcox, Inorg. Chem. 39, 3057 (2000).
D. E. Wilcox, Inorg. Chim. Acta 361, 857 (2008).
Use of Isothermal Titration Calorimetry to Measure Enzyme Kinetic Parameters (Microcal, Northampton, 2004).
G. V. Kotelnikov, S. P. Moiseyeva, E. V. Mezhburd, and V. P. Krayev, J. Therm. Anal. Calorim. 62, 39 (2000).
G. V. Kotelnikov, S. P. Moiseyeva, E. V. Mezhburd, and V. P. Krayev, J. Therm. Anal. Calorim. 68, 803 (2002).
G. V. Kotelnikov, S. P. Moiseyeva, E. V. Mezhburd, et al., J. Therm. Anal. Calorim. 81, 255 (2005).
G. V. Kotel’nikov, S. P. Moiseeva, V. Ya. Grinberg, et al., RF Patent No. 2335743, Byull. Izobret., No. 28 (2008).
G. V. Kotel’nikov, S. P. Moiseeva, V. Ya. Grinberg, et al., RF Patent No. 2335744, Byull. Izobret., No. 28 (2008).
L. E. Briggner and I. Wadso, J. Biochem. Biophys. Methods 22, 101 (1991).
A. Laukkanen, L. Valtola, F. M. Winnik, and H. Tenhu, Macromolecules 37, 2268 (2004).
Y. Maeda, T. Nakamura, and I. Ikeda, Macromolecules 35, 217 (2002).
Ch. Kantor and P. Schimmel, Biophysical Chemistry (Freeman, San Francisco, 1980; Mir, Moscow, 1985).
I. Prigogine and R. Defay, Chemical Thermodynamics (Longmans, London, 1954; Nauka, Novosibirsk, 1966).
A. V. Berezkin, P. G. Khalatur, A. R. Khokhlov, and P. Reineker, New J. Phys. 6, 44 (2004).
A. R. Khokhlov, Macromol. Symp. 143, 207 (1999).
A. R. Khokhlov and P. G. Khalatur, Curr. Opin. Colloid Interface Sci. 10, 22 (2005).
R. Breslow, Pure Appl. Chem. 66, 1573 (1994).
R. Breslow, Pure Appl. Chem. 70, 267 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.V. Burova, N.V. Grinberg, V.I. Lozinskii, S.P. Moiseeva, G.V. Kotel’nikov, V.Ya. Grinberg, A.R. Khokhlov, 2010, published in Vysokomolekulyarnye Soedineniya, Ser. A, 2010, Vol. 52, No. 4, pp. 554–560.
This work was supported by the Russian Foundation for Basic Research, project no. 06-08-01237a, and by the Division of Chemistry and Materials Science, Russian Academy of Sciences, under the program Development and Study of Macromolecules and Macromolecular Structures of New Generations, project Synthesis, Characteristics, and Catalytic Activity of Copolymers Whose Macromolecules Possess Protein-like Conformations in Aqueous Media (Synthetic Enzymes).
Rights and permissions
About this article
Cite this article
Burova, T.V., Grinberg, N.V., Lozinskii, V.I. et al. Energetics of the binding of Cu(II) ions by thermosensitive copolymers of N-vinylcaprolactam and N-vinylimidazole in different conformational states of macromolecules. Polym. Sci. Ser. A 52, 356–361 (2010). https://doi.org/10.1134/S0965545X10040024
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X10040024